Deck 23: Catalysis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 23: Catalysis
1
Describe the first step of a specific-base-catalyzed dehydration of a hydrate.
A)base abstracts a proton, electron pair from O-H bond goes to oxygen
B)negative charge from oxygen forms double bond, hydroxide departs as leaving group
C)base abstracts a proton, electron pair from O-H bond forms a double bond, hydroxide departs as leaving group
D)hydroxide departs as leaving group, then negative charge from oxygen forms a double bond
E)electron pair from O-H bond forms a double bond, base abstracts a proton
A)base abstracts a proton, electron pair from O-H bond goes to oxygen
B)negative charge from oxygen forms double bond, hydroxide departs as leaving group
C)base abstracts a proton, electron pair from O-H bond forms a double bond, hydroxide departs as leaving group
D)hydroxide departs as leaving group, then negative charge from oxygen forms a double bond
E)electron pair from O-H bond forms a double bond, base abstracts a proton
base abstracts a proton, electron pair from O-H bond goes to oxygen
2
Describe the second step of a specific-base-catalyzed dehydration of a hydrate.
A)base abstracts a proton, electron pair from O-H bond goes to oxygen
B)negative charge from oxygen forms double bond, hydroxide departs as leaving group
C)base abstracts a proton, electron pair from O-H bond forms a double bond, hydroxide departs as leaving group
D)hydroxide departs as leaving group, then negative charge from oxygen forms a double bond
E)electron pair from O-H bond forms a double bond, base abstracts a proton
A)base abstracts a proton, electron pair from O-H bond goes to oxygen
B)negative charge from oxygen forms double bond, hydroxide departs as leaving group
C)base abstracts a proton, electron pair from O-H bond forms a double bond, hydroxide departs as leaving group
D)hydroxide departs as leaving group, then negative charge from oxygen forms a double bond
E)electron pair from O-H bond forms a double bond, base abstracts a proton
negative charge from oxygen forms double bond, hydroxide departs as leaving group
3
Describe the reaction coordinate diagram for a specific-acid-catalyzed reaction.
A)one peak
B)two peaks, second peak is larger
C)two peaks, second peak is smaller
D)two peaks, equal size
E)three peaks, middle peak is larger
A)one peak
B)two peaks, second peak is larger
C)two peaks, second peak is smaller
D)two peaks, equal size
E)three peaks, middle peak is larger
two peaks, second peak is larger
4
Describe the acid-catalyzed first slow step in the hydrolysis of an ester.
A)The electron pair on oxygen forms double bond and alcohol is the leaving group.
B)The base abstracts extra hydrogen from protonated alcohol.
C)The oxygen of the carbonyl group abstracts a hydrogen from HB.
D)Water attacks the carbonyl carbon and electron pair goes to oxygen.
E)The oxygen on the alkoxy group abstracts a hydrogen from HB.
A)The electron pair on oxygen forms double bond and alcohol is the leaving group.
B)The base abstracts extra hydrogen from protonated alcohol.
C)The oxygen of the carbonyl group abstracts a hydrogen from HB.
D)Water attacks the carbonyl carbon and electron pair goes to oxygen.
E)The oxygen on the alkoxy group abstracts a hydrogen from HB.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Describe the reaction coordinate diagram for a general-acid-catalyzed reaction.
A)one peak
B)two peaks, second peak is larger
C)two peaks, second peak is smaller
D)two peaks, equal size
E)three peaks, middle peak is larger
A)one peak
B)two peaks, second peak is larger
C)two peaks, second peak is smaller
D)two peaks, equal size
E)three peaks, middle peak is larger

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
How does a base catalyst increase the rate of reaction?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
The reaction below occurs by a general-acid catalyzed mechanism. Use curved arrows to illustrate the ring-forming step of the mechanism. 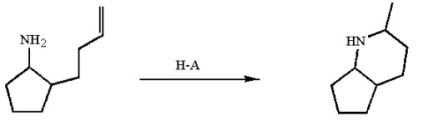


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
Provide two ways in which acid catalyzes ester hydrolysis.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Describe the acid-catalyzed second slow step in the hydrolysis of an ester.
A)The electron pair on oxygen forms double bond and alcohol is the leaving group.
B)The base abstracts extra hydrogen from protonated alcohol.
C)The oxygen of the carbonyl group abstracts a hydrogen from HB.
D)Water attacks the carbonyl carbon and electron pair goes to oxygen.
E)The oxygen on the alkoxy group abstracts a hydrogen from HB.
A)The electron pair on oxygen forms double bond and alcohol is the leaving group.
B)The base abstracts extra hydrogen from protonated alcohol.
C)The oxygen of the carbonyl group abstracts a hydrogen from HB.
D)Water attacks the carbonyl carbon and electron pair goes to oxygen.
E)The oxygen on the alkoxy group abstracts a hydrogen from HB.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements concerning general-acid catalysis is (are)correct?
A)The proton is transferred to the reactant during the slow step of the reaction.
B)The transition state of the slow step of the reaction has a partially transferred proton instead of a fully transferred proton.
C)Typically, a general-acid catalyst is stronger than a specific-acid catalyst.
D)both A and B
E)all of the above
A)The proton is transferred to the reactant during the slow step of the reaction.
B)The transition state of the slow step of the reaction has a partially transferred proton instead of a fully transferred proton.
C)Typically, a general-acid catalyst is stronger than a specific-acid catalyst.
D)both A and B
E)all of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
An acid catalyst functions by donating ________ to a substrate.
A)a proton
B)an electron
C)an electron pair
D)a nucleophile
E)a molecule of water
A)a proton
B)an electron
C)an electron pair
D)a nucleophile
E)a molecule of water

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is/are true about base-catalysts?
A)They increase the rate of a reaction by removing a proton from a reactant.
B)They increase the rate of a reaction by providing a pathway with a more stable transition state.
C)They facilitate the formation of a negatively charged tetrahedral intermediate.
D)A and B only
E)A, B and C
A)They increase the rate of a reaction by removing a proton from a reactant.
B)They increase the rate of a reaction by providing a pathway with a more stable transition state.
C)They facilitate the formation of a negatively charged tetrahedral intermediate.
D)A and B only
E)A, B and C

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Propose a step-by-step mechanism for the general-acid catalyzed reaction shown below: 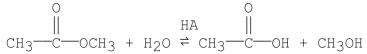
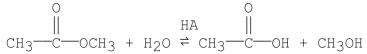

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
List four common types of catalysts used in organic reactions and give one example of each.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
How does an acid catalyst speed up the reaction below? 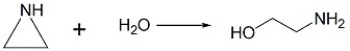
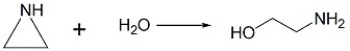

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
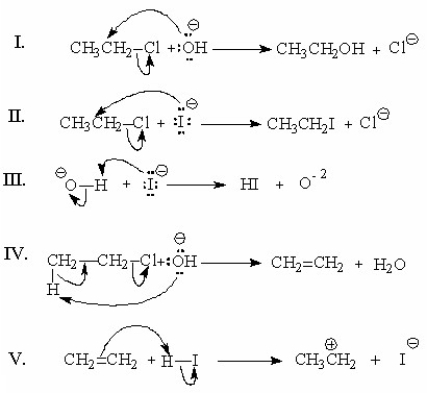
Which of the following represents the first step(s)in the mechanism involving general-base catalysis for the reaction shown below? 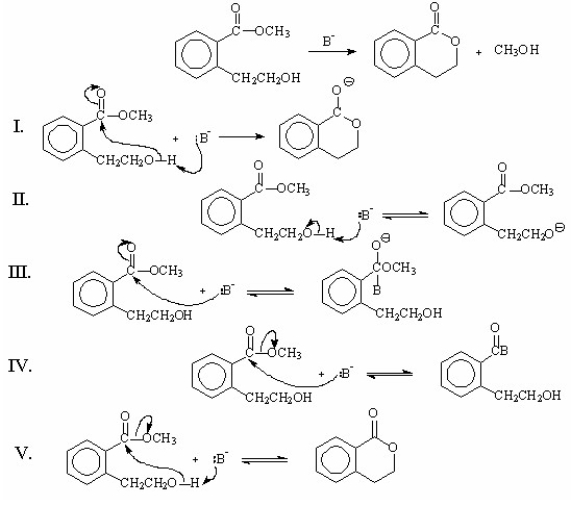
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements about how acid catalyzes the hydrolysis of esters is (are)correct?
A)The acid catalyst increases the rate of formation of the tetrahedral intermediate by protonating the carbonyl and thereby increasing the reactivity of the carbonyl group.
B)The acid catalyst increases the rate of the reaction by changing the basicity of the group eliminated when the tetrahedral intermediate collapses.
C)The acid catalyst protonates the water, the nucleophile in the reaction, and thereby makes it a stronger nucloephile.
D)both A and B
E)all of the above
A)The acid catalyst increases the rate of formation of the tetrahedral intermediate by protonating the carbonyl and thereby increasing the reactivity of the carbonyl group.
B)The acid catalyst increases the rate of the reaction by changing the basicity of the group eliminated when the tetrahedral intermediate collapses.
C)The acid catalyst protonates the water, the nucleophile in the reaction, and thereby makes it a stronger nucloephile.
D)both A and B
E)all of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
How does an acid catalyst increase the rate of reaction?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
In what kind of acid catalysis is the proton transferred completely to the reactant before a subsequent slow step?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is an intermediate in the mechanism of hydrolysis of the ester shown below? 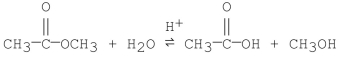
A)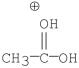
B)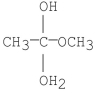
C)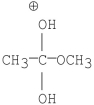
D)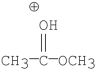
E)all of the above
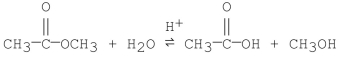
A)

B)

C)

D)

E)all of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
Explain why an intramolecular reaction resulting in the formation of five- or six-membered rings occurs more readily than the corresponding intermolecular reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a step in the mechanism of the reaction shown below? 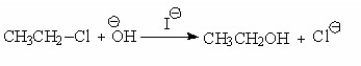
A)I
B)II
C)III
D)IV
E)V


A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
Describe the first step of a general-base-catalyzed dehydration of a hydrate.
A)base abstracts a proton, electron pair from O-H bond goes to oxygen
B)negative charge from oxygen forms double bond, hydroxide departs as leaving group
C)base abstracts a proton, electron pair from O-H bond forms a double bond, hydroxide departs as leaving group
D)hydroxide departs as leaving group, then negative charge from oxygen forms a double bond
E)electron pair from O-H bond forms a double bond, base abstracts a proton
A)base abstracts a proton, electron pair from O-H bond goes to oxygen
B)negative charge from oxygen forms double bond, hydroxide departs as leaving group
C)base abstracts a proton, electron pair from O-H bond forms a double bond, hydroxide departs as leaving group
D)hydroxide departs as leaving group, then negative charge from oxygen forms a double bond
E)electron pair from O-H bond forms a double bond, base abstracts a proton

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
In the hydrolysis of methyl trifluoroacetate, give two purposes of the zinc catalyst.
A)increases the rate of the first step by providing a metal-bound hydroxide ion
B)increases the rate of the second step by decreasing the basicity of the leaving group
C)increases the rate of the second step by increasing the basicity of the leaving group
D)increases the rate of the first step by providing a metal-bound water molecule
E)increases the rate of the last step by replacing alkoxide with hydroxide
A)increases the rate of the first step by providing a metal-bound hydroxide ion
B)increases the rate of the second step by decreasing the basicity of the leaving group
C)increases the rate of the second step by increasing the basicity of the leaving group
D)increases the rate of the first step by providing a metal-bound water molecule
E)increases the rate of the last step by replacing alkoxide with hydroxide

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
The compound CH3OCH2CH2CH2CH2Br undergoes a solvolysis reaction in aqueous ethanol solution. Which of the following is evidence that a neighboring group effect is occurring?
A)CH3CH2CH2CH2Br reacts more slowly than CH3OCH2CH2CH2CH2Br does.
B)The reaction is faster when the concentration of water is higher.
C)The reaction is first order.
D)HBr is a product of the reaction.
A)CH3CH2CH2CH2Br reacts more slowly than CH3OCH2CH2CH2CH2Br does.
B)The reaction is faster when the concentration of water is higher.
C)The reaction is first order.
D)HBr is a product of the reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Give the halide that is the most reactive as a nucleophilic catalyst.
A)iodide
B)bromide
C)chloride
D)fluoride
E)All react equally.
A)iodide
B)bromide
C)chloride
D)fluoride
E)All react equally.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
What factor(s)would affect the rate of an intramolecular reaction?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
Show why typical metal-bound water has a considerably lower pKa than that of water.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
Rank the following compounds in increasing order of reactivity in the intramolecular displacement of p-bromophenolate to form a cyclic anhydride. Explain your ranking. 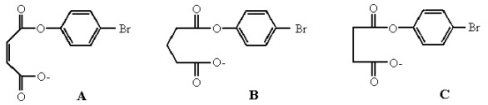


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
"Being in the right place... can be the most powerful factor of all in determining how fast a reaction goes." In terms of reaction rate theory, this statement refers to the ________ for a reaction.
A)probability factor
B)energy factor
C)resonance effect
D)inductive effect
A)probability factor
B)energy factor
C)resonance effect
D)inductive effect

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
Provide the two general ways that metal ions catalyze organic reactions.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following are affected when an intramolecular catalyst is used?
A)number of collisions between the two per unit time
B)fraction of collisions with sufficient energy
C)fraction of collisions with proper orientation
D)rate of the reaction
E)all of the above
A)number of collisions between the two per unit time
B)fraction of collisions with sufficient energy
C)fraction of collisions with proper orientation
D)rate of the reaction
E)all of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is not true about metal-ion catalysts?
A)They coordinate with atoms with nonbonded electrons.
B)They can make a reaction center more susceptible to receiving electrons and stabilize a developing charge on the transition state.
C)They can make a leaving group a weaker base and therefore a better leaving group.
D)They act as Lewis bases.
E)They can increase the electrophilicity of a reaction center.
A)They coordinate with atoms with nonbonded electrons.
B)They can make a reaction center more susceptible to receiving electrons and stabilize a developing charge on the transition state.
C)They can make a leaving group a weaker base and therefore a better leaving group.
D)They act as Lewis bases.
E)They can increase the electrophilicity of a reaction center.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is an intermediate in the metal-ion catalyzed reaction shown below? 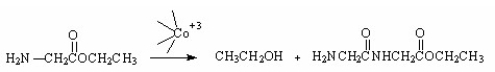
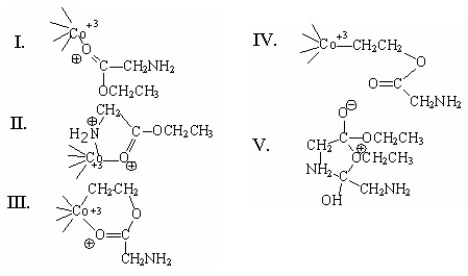
A)I
B)II
C)III
D)IV
E)V
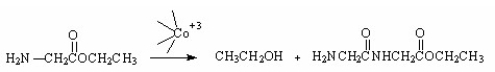

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
How does a metal catalyst increase the rate of reaction?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
How does Zn2+ catalyze the hydrolysis of trifluoroacetate esters?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
Offer an explanation of how iodide catalyzes the hydrolysis of 1-chloropropane in aqueous base.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
What is the main purpose for using metal-ions as catalysts in the following hydrolysis reaction? 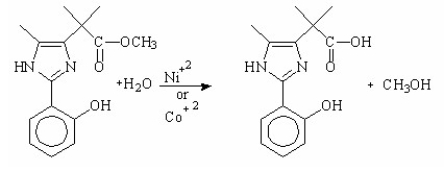
A)increases the rate by making water a stronger base, thereby increasing its electrophilicity
B)increases the rate by making water a stronger base, thereby increasing its nucleophilicity
C)increases the rate by making water a stronger acid, thereby increasing its nucleophilicity
D)increases the rate by making water a stronger acid, thereby increasing its electrophilicity
E)increases the rate by making water a stronger acid, thereby decreasing its nucleophilicity

A)increases the rate by making water a stronger base, thereby increasing its electrophilicity
B)increases the rate by making water a stronger base, thereby increasing its nucleophilicity
C)increases the rate by making water a stronger acid, thereby increasing its nucleophilicity
D)increases the rate by making water a stronger acid, thereby increasing its electrophilicity
E)increases the rate by making water a stronger acid, thereby decreasing its nucleophilicity

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
Hydrolysis of esters occurs more rapidly in the presence of catalytic imidazole. Explain this phenomenon and provide the structure of the acyl imidazole intermediate which is formed in the catalyzed process.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
How does Cu2+ catalyze the decarboxylation of dimethyloxaloacetate?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is not true about serine proteases?
A)They are called serine proteases because they are able to break the peptide chain at the serine linkage.
B)Endopeptidases and serine proteases are synonyms with each other.
C)Trypsin, chymotrypsin, and elastase are all serine proteases.
D)Serine proteases catalyze the hydrolysis of protein peptide chains.
E)They are called serine proteases because they all have a serine residue that participates in the catalytic process.
A)They are called serine proteases because they are able to break the peptide chain at the serine linkage.
B)Endopeptidases and serine proteases are synonyms with each other.
C)Trypsin, chymotrypsin, and elastase are all serine proteases.
D)Serine proteases catalyze the hydrolysis of protein peptide chains.
E)They are called serine proteases because they all have a serine residue that participates in the catalytic process.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
Explain the phenomenon known as molecular recognition. Give an example.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is true of globular proteins?
A)They may be proteins with predominantly quaternary structure.
B)They may be circular strands of short peptides.
C)They may be three-dimensional chains of short peptides.
D)They may be enzymes that catalyze biological reactions.
E)They may be large strands of proteins and amylopectins.
A)They may be proteins with predominantly quaternary structure.
B)They may be circular strands of short peptides.
C)They may be three-dimensional chains of short peptides.
D)They may be enzymes that catalyze biological reactions.
E)They may be large strands of proteins and amylopectins.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the labeled bonds in the following peptide chain will break when carboxypeptidase A is used to hydrolyze the chain? 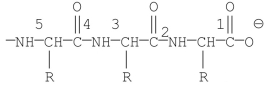
A)1
B)2
C)3
D)4
E)5
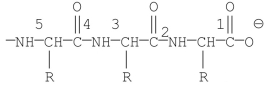
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
Give the starting materials for the following products. 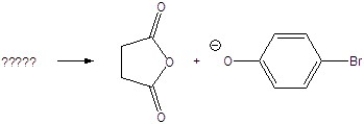


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Which ester below hydrolyzes more readily and why? 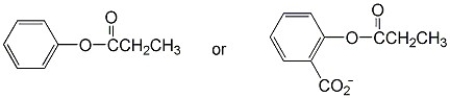


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is not true about the enzyme carboxypeptidase A?
A)Arg 145 and Tyr 248 are involved in the catalytic process of the enzyme.
B)The metal ion in carboxypeptidase A is partially liganded to the oxygen of the carbonyl group that will be hydrolyzed.
C)Carboxypeptidase A catalyzes the hydrolysis of the C-terminal peptide bond in proteins.
D)Carboxypeptidase A is a metalloenzyme.
E)The metal ion in carboxypeptidase is a Cu+2.
A)Arg 145 and Tyr 248 are involved in the catalytic process of the enzyme.
B)The metal ion in carboxypeptidase A is partially liganded to the oxygen of the carbonyl group that will be hydrolyzed.
C)Carboxypeptidase A catalyzes the hydrolysis of the C-terminal peptide bond in proteins.
D)Carboxypeptidase A is a metalloenzyme.
E)The metal ion in carboxypeptidase is a Cu+2.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
Give the product(s)for the following reaction. 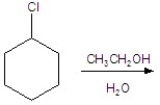
A)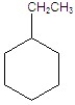
B)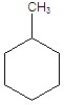
C)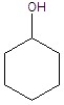
D)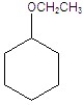
E)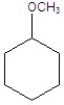

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
________ molarity is the concentration of the reactant that would be required to give the corresponding intermolecular reaction the same rate as its intramolecular counterpart.
A)Effective
B)Entropic
C)Enthalpic
D)Hyperionic
E)Deficient
A)Effective
B)Entropic
C)Enthalpic
D)Hyperionic
E)Deficient

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
Give the products of the following reaction. 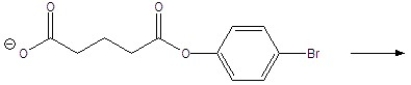


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
Explain what is meant by anchimeric assistance and give an example.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
Rank the following alkyl chlorides in increasing order of reactivity in aqueous ethanol, and explain why one of the compounds is 70,000 times more reactive than the other two. 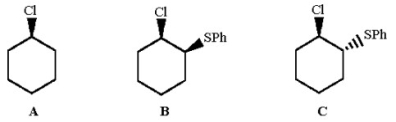


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
A pure sample of the trans-2-bromocyclopentanol enantiomer shown below reacts with HCl. The reaction proceeds by way of a bromonium ion. What is/are the product(s)of the reaction? 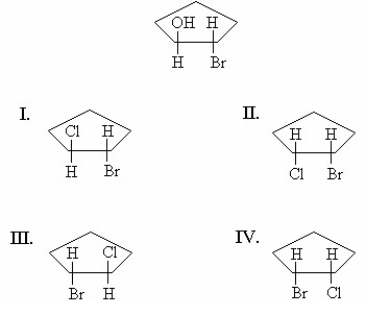
A)I only
B)II only
C)I and III
D)II and IV

A)I only
B)II only
C)I and III
D)II and IV

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements account for the remarkable catalytic ability of enzymes?
A)proper positioning and specificity of active site and substrate
B)proper positioning of amino acid side chains that serve as catalytic groups
C)ability of the groups on the enzyme to stabilize transition states
D)A and B
E)all of the above
A)proper positioning and specificity of active site and substrate
B)proper positioning of amino acid side chains that serve as catalytic groups
C)ability of the groups on the enzyme to stabilize transition states
D)A and B
E)all of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
Explain the large difference in the relative rates of the following hydrolysis reactions: 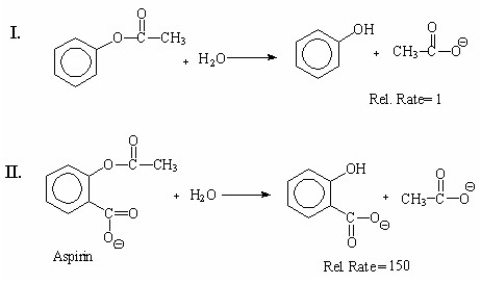


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is the main difference among the active sites of all serine proteases?
A)The presence of serine residue at the active site
B)The size and charge of the pocket at the active site
C)The presence of histidine residue at the active site
D)The presence of aspartate at the active site
E)The hydrolysis of the peptide bond
A)The presence of serine residue at the active site
B)The size and charge of the pocket at the active site
C)The presence of histidine residue at the active site
D)The presence of aspartate at the active site
E)The hydrolysis of the peptide bond

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
How do enzymes differ from nonbiological catalysts?
A)They have specificity for the substrate.
B)They have lower molecular weights than nonbiological catalysts.
C)They require higher pressure to be functional.
D)They require lower pH to be functional.
E)They are more polar than nonbiological catalysts.
A)They have specificity for the substrate.
B)They have lower molecular weights than nonbiological catalysts.
C)They require higher pressure to be functional.
D)They require lower pH to be functional.
E)They are more polar than nonbiological catalysts.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
What does the model termed "lock-and-key" stand for?
A)The hydrogen bonds in the α-helix fit each other like a key fits a lock.
B)The amino acids in a peptide chain fit each other like a key fits a lock.
C)The substrate fits the active site of the enzyme like a key fits a lock.
D)The nonpolar sites of proteins fit each other like a key fits a lock.
E)The N-terminal and the C-terminal ends of a protein fit each other like a key fits a lock.
A)The hydrogen bonds in the α-helix fit each other like a key fits a lock.
B)The amino acids in a peptide chain fit each other like a key fits a lock.
C)The substrate fits the active site of the enzyme like a key fits a lock.
D)The nonpolar sites of proteins fit each other like a key fits a lock.
E)The N-terminal and the C-terminal ends of a protein fit each other like a key fits a lock.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
Explain what is meant by the induced fit model. Give an example.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following sets of amino acids is found in all serine proteases?
A)arginine, histidine, serine
B)arginine, aspartate, serine
C)proline, histidine, serine
D)aspartate, histidine, serine
E)aspartate, proline, serine
A)arginine, histidine, serine
B)arginine, aspartate, serine
C)proline, histidine, serine
D)aspartate, histidine, serine
E)aspartate, proline, serine

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
Give the final step for the mechanism for glucose-6-phosphate isomerase.
A)In the ring opening reaction, a base catalyst removes a proton from the OH group and an acid catalyst aids in the departure of the leaving group by protonating it.
B)A base catalyst removes a proton from the a-carbon of the aldehyde.
C)The enol is converted to a ketone.
D)The ester group is hydrolyzed.
E)The conjugate base of the acid catalyst and the conjugate acid of the base catalyst catalyze ring closure.
A)In the ring opening reaction, a base catalyst removes a proton from the OH group and an acid catalyst aids in the departure of the leaving group by protonating it.
B)A base catalyst removes a proton from the a-carbon of the aldehyde.
C)The enol is converted to a ketone.
D)The ester group is hydrolyzed.
E)The conjugate base of the acid catalyst and the conjugate acid of the base catalyst catalyze ring closure.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
Explain what is meant by site-specific mutagenesis.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following residues acts as a general-base catalyst in the cleavage of the C3-C4 bond in the mechanism of the aldolase-catalyzed reaction?
A)methionine
B)histidine
C)cysteine
D)asparagine
E)lysine
A)methionine
B)histidine
C)cysteine
D)asparagine
E)lysine

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the factors that contribute to the catalytic ability of enzymes.
A)proper orientation of the reacting groups
B)some amino acid side chain groups of the enzyme serve as catalysts
C)some amino acid side chains stabilize transition states and intermediates
D)metal ions in enzymes serve as catalysts
E)all of the above
A)proper orientation of the reacting groups
B)some amino acid side chain groups of the enzyme serve as catalysts
C)some amino acid side chains stabilize transition states and intermediates
D)metal ions in enzymes serve as catalysts
E)all of the above

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements best describes what lysozyme is?
A)an enzyme that destroys the cell walls of bacteria
B)an enzyme that repairs the cell walls of bacteria
C)an enzyme that hydrolyzes the NAM-NAG bond
D)A and C
E)B and C
A)an enzyme that destroys the cell walls of bacteria
B)an enzyme that repairs the cell walls of bacteria
C)an enzyme that hydrolyzes the NAM-NAG bond
D)A and C
E)B and C

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Give the first step for the mechanism for serine protease.
A)Histidine functions as a base , increasing the nucleophilicity of serine, which attacks the carbonyl group.
B)The tetrahedral complex collapses, expelling the amino group.
C)Water attacks the acyl group of the acyl-enzyme intermediate.
D)The tetrahedral intermediate collapses, expelling serine.
E)The negatively charged oxygen hydrogen bonds with two peptide bonds.
A)Histidine functions as a base , increasing the nucleophilicity of serine, which attacks the carbonyl group.
B)The tetrahedral complex collapses, expelling the amino group.
C)Water attacks the acyl group of the acyl-enzyme intermediate.
D)The tetrahedral intermediate collapses, expelling serine.
E)The negatively charged oxygen hydrogen bonds with two peptide bonds.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
What are NAM and NAG and what do they do?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
Why is histidine a versatile catalytic group?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
The enzyme lysozyme exhibits greatest activity in solutions where the pH is maintained between 3.8 and 6.7. Which of the following conclusions about the Asp 52 and Glu 35 residues in the enzyme's active site can be reached?
A)Lysozyme is maximally active when both Asp 52 and Glu 35 are in their acidic forms.
B)Lysozyme is maximally active when both Asp 52 and Glu 35 are in their basic forms.
C)Lysozyme is maximally active when both Asp 52 and Glu 35 are in their neutral forms.
D)Lysozyme is maximally active when Asp 52 is in its acidic form and Glu 35 is in its basic form.
E)Lysozyme is maximally active when Asp 52 is in its basic form and Glu 35 is in its acidic form.
A)Lysozyme is maximally active when both Asp 52 and Glu 35 are in their acidic forms.
B)Lysozyme is maximally active when both Asp 52 and Glu 35 are in their basic forms.
C)Lysozyme is maximally active when both Asp 52 and Glu 35 are in their neutral forms.
D)Lysozyme is maximally active when Asp 52 is in its acidic form and Glu 35 is in its basic form.
E)Lysozyme is maximally active when Asp 52 is in its basic form and Glu 35 is in its acidic form.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
Which amino acid component is most likely to serve as a proton donor in an enzyme's active site?
A)methionine
B)glutamic acid
C)valine
D)phenylalanine
E)pyroline
A)methionine
B)glutamic acid
C)valine
D)phenylalanine
E)pyroline

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
Stabilization of a charge by an opposite charge is called ________ catalysis.
A)electrostatic
B)osmotic
C)substrate
D)activated
E)covalent
A)electrostatic
B)osmotic
C)substrate
D)activated
E)covalent

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following residues acts as a general-acid catalyst in aldolase but as a general-base catalyst in chymotrypsin?
A)methionine
B)histidine
C)cysteine
D)asparagine
E)lysine
A)methionine
B)histidine
C)cysteine
D)asparagine
E)lysine

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
Give the first step of the mechanism for aldolase.
A)Fructose-1,6,-diphosphate forms an imine with a lysine residue at the active site of the enzyme.
B)A tyrosine residue functions as a base catalyst to cleave the bond between C-3 and C-4.
C)The enamine intermediate rearranges to an imine.
D)Hydrolysis of the imine releases dihydroxyacetone phosphate.
E)A molecule of glyceraldehyde-3-phosphate is expelled.
A)Fructose-1,6,-diphosphate forms an imine with a lysine residue at the active site of the enzyme.
B)A tyrosine residue functions as a base catalyst to cleave the bond between C-3 and C-4.
C)The enamine intermediate rearranges to an imine.
D)Hydrolysis of the imine releases dihydroxyacetone phosphate.
E)A molecule of glyceraldehyde-3-phosphate is expelled.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following intermediates is produced in the second step of the mechanism for bovine chymotrypsin hydrolysis of a peptide bond?
A)epoxy-enzyme intermediate
B)carboxy-enzyme intermediate
C)acyl-enzyme intermediate
D)phenoxy-enzyme intermediate
E)oxy-enzyme intermediate
A)epoxy-enzyme intermediate
B)carboxy-enzyme intermediate
C)acyl-enzyme intermediate
D)phenoxy-enzyme intermediate
E)oxy-enzyme intermediate

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
The specificity of an enzyme for its substrate is an example of ________.
A)molecular recognition
B)the anomeric effect
C)specific-acid catalysis
D)general-acid catalysis
E)a pericyclic reaction
A)molecular recognition
B)the anomeric effect
C)specific-acid catalysis
D)general-acid catalysis
E)a pericyclic reaction

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following residues are found at the active site of lysozyme?
A)His 57, Asp 102
B)His 57, Asp 52
C)Glu 35, His 57
D)Asp 102, Glu 35
E)Glu 35, Asp 52
A)His 57, Asp 102
B)His 57, Asp 52
C)Glu 35, His 57
D)Asp 102, Glu 35
E)Glu 35, Asp 52

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
Which amino acid component of an enzyme has a chain that can form an imine with the substrate?
A)lysine
B)methionine
C)phenylalanine
D)serine
E)aspartic acid
A)lysine
B)methionine
C)phenylalanine
D)serine
E)aspartic acid

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is not true about the enzyme aldolase?
A)It catalyzes the cleavage of D-glucose into two molecules each containing 3-carbons.
B)It catalyzes the cleavage of D-galactose into two molecules each containing 3-carbons.
C)It catalyzes the cleavage of a six carbon compound into two three carbon compounds.
D)It catalyzes the cleavage of D-fructose 1,6-diphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
E)The enzyme is called aldolase because the reverse reaction is an aldol condensation.
A)It catalyzes the cleavage of D-glucose into two molecules each containing 3-carbons.
B)It catalyzes the cleavage of D-galactose into two molecules each containing 3-carbons.
C)It catalyzes the cleavage of a six carbon compound into two three carbon compounds.
D)It catalyzes the cleavage of D-fructose 1,6-diphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
E)The enzyme is called aldolase because the reverse reaction is an aldol condensation.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
What technique is used to gain information about protein structure and function by replacing single amino acids along a peptide chain?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
After lysozyme binds its substrate, the enzyme undergoes a conformational change that distorts residue D in the substrate. Which of the following best describes this distortion?
A)from a chair to a half-chair conformation
B)from a half-chair to a chair conformation
C)from a chair to a boat conformation
D)from a boat to a chair conformation
E)from a boat to a half-chair conformation
A)from a chair to a half-chair conformation
B)from a half-chair to a chair conformation
C)from a chair to a boat conformation
D)from a boat to a chair conformation
E)from a boat to a half-chair conformation

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck


