Deck 17: Thermodynamics: Entropy, free Energy, and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/150
Play
Full screen (f)
Deck 17: Thermodynamics: Entropy, free Energy, and Equilibrium
1
Predict the sign of ΔS for each of the following processes,which occur at constant temperature. I.The volume of 2.0 moles of O2(g)increases from 44 L to 52 L.
II.The pressure of 2.0 moles of O2(g)increases from 1.0 atm to 1.2 atm.
A)I: ΔS = negative;II: ΔS = negative
B)I: ΔS = negative;II: ΔS = positive
C)I: ΔS = positive;II: ΔS = negative
D)I: ΔS = positive;II: ΔS = positive
II.The pressure of 2.0 moles of O2(g)increases from 1.0 atm to 1.2 atm.
A)I: ΔS = negative;II: ΔS = negative
B)I: ΔS = negative;II: ΔS = positive
C)I: ΔS = positive;II: ΔS = negative
D)I: ΔS = positive;II: ΔS = positive
I: ΔS = positive;II: ΔS = negative
2
Classify each of the following processes as spontaneous or nonspontaneous. I.H2O(l)→ H2O(g)T = 25°C,vessel open to atmosphere with 50% relative humidity
II.H2O(s)→ H2O(l)T = 25°C,P = 1 atm
A)I and II are both spontaneous.
B)I is spontaneous and II is nonspontaneous.
C)I is nonspontaneous and II is spontaneous.
D)I and II are both nonspontaneous.
II.H2O(s)→ H2O(l)T = 25°C,P = 1 atm
A)I and II are both spontaneous.
B)I is spontaneous and II is nonspontaneous.
C)I is nonspontaneous and II is spontaneous.
D)I and II are both nonspontaneous.
I and II are both spontaneous.
3
What is the entropy change associated with the expansion of one mole of an ideal gas from an initial volume of V to a final volume of V of 2.50V at constant temperature?
A)ΔS = 2.50 R ln (Vf/Vi)
B)ΔS = -2.50 R ln (Vf/Vi)
C)ΔS = R ln 2.50
D)ΔS = -R ln 2.50
A)ΔS = 2.50 R ln (Vf/Vi)
B)ΔS = -2.50 R ln (Vf/Vi)
C)ΔS = R ln 2.50
D)ΔS = -R ln 2.50
ΔS = R ln 2.50
4
The reaction A(g)→ B(g)is spontaneous under standard conditions.Which of the following statements must be true? I.The reaction B(g)→ A(g)is nonspontaneous under standard conditions.
II.A(g)will be completely converted to B(g)if sufficient time is allowed.
III.A(g)will be completely converted to B(g)rapidly.
A)none of these
B)I
C)I and II
D)I,II,and III
II.A(g)will be completely converted to B(g)if sufficient time is allowed.
III.A(g)will be completely converted to B(g)rapidly.
A)none of these
B)I
C)I and II
D)I,II,and III

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following processes are spontaneous? I.dissolving more solute in an unsaturated solution
II.dissolving more solute in a saturated solution
III.dissolving more solute in a supersaturated solution
A)none of these
B)I
C)I and II
D)I,II,and III
II.dissolving more solute in a saturated solution
III.dissolving more solute in a supersaturated solution
A)none of these
B)I
C)I and II
D)I,II,and III

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
6
Assume a heteronuclear diatomic molecule,AB,forms a one-dimensional crystal by lining up along the x-axis.Also assume that each molecule can only have one of six possible orientations,corresponding to atom A facing in either the positive or negative direction along the x-,y-,or z-axis.If the molecules are arranged randomly in the six directions,the molar entropy at absolute zero should be
A)R ln 6.
B)R ln 66.
C)R ln 6!
D)0.
A)R ln 6.
B)R ln 66.
C)R ln 6!
D)0.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
7
What is k in Boltzmann's formula,S = k ln W?
A)the degeneracy of the state
B)the equilibrium constant for the process
C)the universal gas constant divided by Avogadro's number
D)the universal gas constant times Avogadro's number
A)the degeneracy of the state
B)the equilibrium constant for the process
C)the universal gas constant divided by Avogadro's number
D)the universal gas constant times Avogadro's number

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements is not true?
A)The reverse of a spontaneous reaction is always nonspontaneous.
B)A spontaneous process always moves toward equilibrium.
C)A nonspontaneous process cannot be caused to occur.
D)A highly spontaneous process need not occur rapidly.
A)The reverse of a spontaneous reaction is always nonspontaneous.
B)A spontaneous process always moves toward equilibrium.
C)A nonspontaneous process cannot be caused to occur.
D)A highly spontaneous process need not occur rapidly.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following processes is non-spontaneous at standard temperature and pressure?
A)Iron metal,oxygen,and water form rust (Fe2O3).
B)Melting of solid ice to ice water.
C)Diffusion of perfume molecules across a room.
D)A reaction at equilibrium moves to non-equilibrium.
A)Iron metal,oxygen,and water form rust (Fe2O3).
B)Melting of solid ice to ice water.
C)Diffusion of perfume molecules across a room.
D)A reaction at equilibrium moves to non-equilibrium.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
10
Which forward reaction is a nonspontaneous process?
A)the expansion of a gas into a vacuum
B)N2(g)+ 3 H2(g)⇌ 2 NH3(g)if PH₂ = PN₂ = 1 atm,PNH₃ = 0,and Kp = 4 × 105
C)2 NH3(g)⇌ N2(g)+ 3 H2(g)if PNH₃ = 1 atm, PH₂ = PN₂ = 0,and Kp = 2 × 10-6
D)None of these
A)the expansion of a gas into a vacuum
B)N2(g)+ 3 H2(g)⇌ 2 NH3(g)if PH₂ = PN₂ = 1 atm,PNH₃ = 0,and Kp = 4 × 105
C)2 NH3(g)⇌ N2(g)+ 3 H2(g)if PNH₃ = 1 atm, PH₂ = PN₂ = 0,and Kp = 2 × 10-6
D)None of these

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
11
Sodium reacts violently with water according to the equation: 2 Na(s)+ 2 H2O(l)→ 2 NaOH(aq)+ H2(g)
The resulting solution has a higher temperature than the water prior to the addition of sodium.What are the signs of ΔH° and ΔS° for this reaction?
A)ΔH° is negative and ΔS° is negative.
B)ΔH° is negative and ΔS° is positive.
C)ΔH° is positive and ΔS° is negative.
D)ΔH° is positive and ΔS° is positive.
The resulting solution has a higher temperature than the water prior to the addition of sodium.What are the signs of ΔH° and ΔS° for this reaction?
A)ΔH° is negative and ΔS° is negative.
B)ΔH° is negative and ΔS° is positive.
C)ΔH° is positive and ΔS° is negative.
D)ΔH° is positive and ΔS° is positive.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
12
The entropy change associated with the expansion of one mole of an ideal gas from an initial volume of Vi to a final volume of Vf at constant temperature is given by the equation,ΔS = R ln (Vf/Vi).What is the entropy change associated with the expansion of three moles of an ideal gas from an initial volume of Vi to a final volume of Vf at constant temperature?
A)ΔS = R ln (Vf/Vi)
B)ΔS = 3 mol × R ln (Vf/Vi)
C)ΔS = R ln (Vf × 23/Vi)
D)ΔS = R ln (Vf × 3!/Vi)
A)ΔS = R ln (Vf/Vi)
B)ΔS = 3 mol × R ln (Vf/Vi)
C)ΔS = R ln (Vf × 23/Vi)
D)ΔS = R ln (Vf × 3!/Vi)

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
13
Entropy is a measure of
A)free energy.
B)the heat of a reaction.
C)molecular randomness.
D)the rate of a reaction.
A)free energy.
B)the heat of a reaction.
C)molecular randomness.
D)the rate of a reaction.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
14
An electron in an oxygen p orbital on which of the following would have the highest entropy?
A)CH3CH2OH
B)CH3CH2O-
C)CH3CO2OH
D)CH3CO2-
A)CH3CH2OH
B)CH3CH2O-
C)CH3CO2OH
D)CH3CO2-

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
15
The brown color associated with photochemical smog is due to NO2(g),which is involved in an equilibrium with N2O4(g)in the atmosphere. 2 NO2(g)⇌ N2O4(g)
Predict the signs of the enthalpy and entropy change for the forward reaction.
A)The enthalpy change is negative and the entropy change is negative.
B)The enthalpy change is negative and the entropy change is positive.
C)The enthalpy change is positive and the entropy change is negative.
D)The enthalpy change is positive and the entropy change is positive.
Predict the signs of the enthalpy and entropy change for the forward reaction.
A)The enthalpy change is negative and the entropy change is negative.
B)The enthalpy change is negative and the entropy change is positive.
C)The enthalpy change is positive and the entropy change is negative.
D)The enthalpy change is positive and the entropy change is positive.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
16
What is the entropy of 105 molecules in 1010 boxes?
A)1.59 × 10-21
B)3.45 × 10-20
C)1.38 × 10-17
D)3.18 × 10-17
A)1.59 × 10-21
B)3.45 × 10-20
C)1.38 × 10-17
D)3.18 × 10-17

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
17
Without doing any calculations,determine whether the standard entropy change,ΔS° is positive or negative for each of the following reactions. reaction 1: C(graphite)+ O2(g)→ CO2(g)
Reaction 2: 2 CO(g)+ O2(g)→ 2 CO2(g)
A)ΔS° is positive for both reactions.
B)ΔS° is positive for reaction 1 but negative for reaction 2.
C)ΔS° is positive for reaction 2 but negative for reaction 1.
D)ΔS° is negative for both reactions.
Reaction 2: 2 CO(g)+ O2(g)→ 2 CO2(g)
A)ΔS° is positive for both reactions.
B)ΔS° is positive for reaction 1 but negative for reaction 2.
C)ΔS° is positive for reaction 2 but negative for reaction 1.
D)ΔS° is negative for both reactions.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
18
What is W in Boltzmann's formula,S = k ln W?
A)a fraction indicating the probability of obtaining a result
B)a random number
C)the number of ways of obtaining the state
D)the work times Avogadro's number
A)a fraction indicating the probability of obtaining a result
B)a random number
C)the number of ways of obtaining the state
D)the work times Avogadro's number

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
19
The Boltzmann formula is S = k ln W.A perfect crystal has a molar entropy of 0 at absolute zero because
A)W = 0.
B)W = 1.
C)W = NA.
D)k = 1.
A)W = 0.
B)W = 1.
C)W = NA.
D)k = 1.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
20
Without doing any calculations,determine whether the standard entropy change,ΔS° is positive or negative for each of the following reactions. reaction 1: S(s,rhombic)+ O2(g)→ SO2(g)
Reaction 2: 2 SO2(g)+ O2(g)→ 2 SO3(g)
A)ΔS° is positive for both reactions.
B)ΔS° is positive for reaction 1 but negative for reaction 2.
C)ΔS° is positive for reaction 2 but negative for reaction 1.
D)ΔS° is negative for both reactions.
Reaction 2: 2 SO2(g)+ O2(g)→ 2 SO3(g)
A)ΔS° is positive for both reactions.
B)ΔS° is positive for reaction 1 but negative for reaction 2.
C)ΔS° is positive for reaction 2 but negative for reaction 1.
D)ΔS° is negative for both reactions.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
21
For the process CaCO3(calcite)→ CaCO3(aragonite)ΔH° = -0.21 kJ,ΔS° = -4.2 J/K
Assuming that the surroundings can be considered a large heat reservoir at 25°C,calculate ΔSsurr and ΔStotal for the process at 25°C and 1 atm pressure.Is the process spontaneous at 25°C and 1 atm pressure?
A)ΔSsurr = 4.2 J/K,Δtotal = 0,not spontaneous
B)ΔSsurr = 0.7 J/K,ΔStotal = -3.5 J/K,not spontaneous
C)ΔSsurr = -0.7 J/K,ΔStotal = -4.9 J/K,spontaneous
D)ΔSsurr = -0.7 J/K,ΔStotal = -4.9 J/K,not spontaneous
Assuming that the surroundings can be considered a large heat reservoir at 25°C,calculate ΔSsurr and ΔStotal for the process at 25°C and 1 atm pressure.Is the process spontaneous at 25°C and 1 atm pressure?
A)ΔSsurr = 4.2 J/K,Δtotal = 0,not spontaneous
B)ΔSsurr = 0.7 J/K,ΔStotal = -3.5 J/K,not spontaneous
C)ΔSsurr = -0.7 J/K,ΔStotal = -4.9 J/K,spontaneous
D)ΔSsurr = -0.7 J/K,ΔStotal = -4.9 J/K,not spontaneous

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
22
Why is the sign of ΔG rather than the sign of ΔStotal generally used to determine the spontaneity of a chemical reaction?
A)ΔG can be used for processes that occur under any conditions.
B)ΔG involves thermodynamic functions of the system only.
C)Free energy is easier to understand than entropy.
D)Entropy is based on probability and is therefore less reliable.
A)ΔG can be used for processes that occur under any conditions.
B)ΔG involves thermodynamic functions of the system only.
C)Free energy is easier to understand than entropy.
D)Entropy is based on probability and is therefore less reliable.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate ΔS° for the following reaction. N2(g)+ 2 O2(g)→ 2 NO2(g) 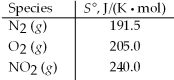
A)-156.5 J/K
B)-121.5 J/K
C)15.5 J/K
D)636.5 J/K

A)-156.5 J/K
B)-121.5 J/K
C)15.5 J/K
D)636.5 J/K

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
24
The solubility of manganese(II)fluoride in water is 6.6 g/mL at 40°C and 4.8 g/L at 100°C.Based on these data,what is the sign of ΔH° and ΔS° for the process below? MnF2(s)⇌ Mn2+(aq)+ 2 F-(aq)
A)ΔH° is negative but the sign of ΔS° cannot be determined from this information.
B)ΔH° is negative and ΔS° is definitely negative.
C)ΔH° is positive but the sign of ΔS° cannot be determined from this information.
D)ΔH° is positive and ΔS° is definitely negative.
A)ΔH° is negative but the sign of ΔS° cannot be determined from this information.
B)ΔH° is negative and ΔS° is definitely negative.
C)ΔH° is positive but the sign of ΔS° cannot be determined from this information.
D)ΔH° is positive and ΔS° is definitely negative.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
25
For bromine,ΔH°vap = 30.91 kJ/mol and ΔS°vap = 93.23 JK-1mol-1 at 25°C.What is the normal boiling point for bromine?
A)25°C
B)58°C
C)124°C
D)332°C
A)25°C
B)58°C
C)124°C
D)332°C

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
26
ΔS° = -198.7 J/K for the reaction shown below.Calculate S° for NH3(g). N2(g)+ 3 H2(g)→ 2 NH3(g) 
A)61.7 J/K∙mol
B)123.4 J/K∙mol
C)192.3 J/K∙mol
D)384.6 J/K∙mol

A)61.7 J/K∙mol
B)123.4 J/K∙mol
C)192.3 J/K∙mol
D)384.6 J/K∙mol

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
27
Which statement is true about the formation of CaCO3(s)from CaO(s)and CO2(g)at 1.00 atm? CaO(s)+ CO2(g)→ CaCO3(s) ΔH° = -178.7 kJ and ΔS° = -150.4 J/K
A)The reaction is spontaneous at all temperatures.
B)The reaction is spontaneous at high temperatures.
C)The reaction is spontaneous at low temperatures.
D)The reaction is not spontaneous at any temperature.
A)The reaction is spontaneous at all temperatures.
B)The reaction is spontaneous at high temperatures.
C)The reaction is spontaneous at low temperatures.
D)The reaction is not spontaneous at any temperature.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate ΔS° for the formation of three moles of solid sodium bromide from the elements at 25°C. 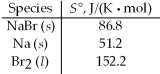
A)-350.1 J/K
B)-243.6 J/K
C)-121.5 J/K
D)260.4 J/K

A)-350.1 J/K
B)-243.6 J/K
C)-121.5 J/K
D)260.4 J/K

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
29
What is the sign of ΔS for each of the following processes? I.The separation of gaseous molecules of UF6,into 238UF6 and 235UF6
At constant temperature and pressure.
II.The dissolving of I2(s)in CCl4(l).
A)ΔS is negative for I and negative for II.
B)ΔS is negative for I and positive for II.
C)ΔS is positive for I and negative for II.
D)ΔS is positive for I and positive for II.
At constant temperature and pressure.
II.The dissolving of I2(s)in CCl4(l).
A)ΔS is negative for I and negative for II.
B)ΔS is negative for I and positive for II.
C)ΔS is positive for I and negative for II.
D)ΔS is positive for I and positive for II.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
30
The standard molar entropy for Br2(g)is 245.46 J/(mol ∙ K)at 25°C.Given that ΔS° = 104.58 J/K for the dissociation of one mole of Br2(g)into Br(g)at 25°C,find the standard molar entropy for Br(g)at 25°C.
A)70.44 J/(mol ∙ K)
B)140.08 J/(mol ∙ K)
C)175.02 J/(mol ∙ K)
D)350.04 J/(mol ∙ K)
A)70.44 J/(mol ∙ K)
B)140.08 J/(mol ∙ K)
C)175.02 J/(mol ∙ K)
D)350.04 J/(mol ∙ K)

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction 3 C2H2(g)→ C6H6(l)at 25°C,the standard enthalpy change is -631 kJ and the standard entropy change is -430 J/K.Calculate the standard free energy change at 25°C.
A)948 kJ
B)-503 kJ
C)-618 kJ
D)-1061 kJ
A)948 kJ
B)-503 kJ
C)-618 kJ
D)-1061 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
32
For a process to be at equilibrium,it is necessary that
A)ΔSsys = ΔSsurr.
B)ΔSsys = -ΔSsurr.
C)ΔSsys = 0.
D)ΔSsys = 0 and ΔSsurr = 0.
A)ΔSsys = ΔSsurr.
B)ΔSsys = -ΔSsurr.
C)ΔSsys = 0.
D)ΔSsys = 0 and ΔSsurr = 0.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
33
At constant pressure and temperature,which statement is true?
A)All reactions for which ΔH < 0 are spontaneous.
B)All reactions for which ΔS < 0 are spontaneous.
C)All reactions for which ΔG < 0 are spontaneous.
D)All reactions for which K < 1 are spontaneous.
A)All reactions for which ΔH < 0 are spontaneous.
B)All reactions for which ΔS < 0 are spontaneous.
C)All reactions for which ΔG < 0 are spontaneous.
D)All reactions for which K < 1 are spontaneous.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements must be true for the entropy of a pure solid to be zero? I.The temperature must be 0 K.
II.The solid must be crystalline,not amorphous.
III.The solid must be perfectly ordered.
IV.The solid must be an element.
A)I
B)I and II
C)I,II,and III
D)I,II,III,and IV
II.The solid must be crystalline,not amorphous.
III.The solid must be perfectly ordered.
IV.The solid must be an element.
A)I
B)I and II
C)I,II,and III
D)I,II,III,and IV

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
35
A hot penny is dropped into cold water inside a polystyrene foam cup.Assuming negligible heat loss to the atmosphere and the cup,
A)the decrease in entropy of the penny is equal to the increase in entropy of the water.
∣ ΔSpenny ∣ = ∣ ΔSwater ∣
B)the decrease in entropy of the penny is less than the increase in entropy of the water.
∣ ΔSpenny ∣ < ∣ ΔSwater ∣
C)the decrease in entropy of the penny is more than the increase in entropy of the water.
∣ ΔSpenny ∣ > ∣ ΔSwater ∣
D)the entropy of both the penny and the water increases.
A)the decrease in entropy of the penny is equal to the increase in entropy of the water.
∣ ΔSpenny ∣ = ∣ ΔSwater ∣
B)the decrease in entropy of the penny is less than the increase in entropy of the water.
∣ ΔSpenny ∣ < ∣ ΔSwater ∣
C)the decrease in entropy of the penny is more than the increase in entropy of the water.
∣ ΔSpenny ∣ > ∣ ΔSwater ∣
D)the entropy of both the penny and the water increases.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the reaction: N2(g)+ 3 F2(g)→ 2 NF3(g) ΔH° = -249 kJ and ΔS° = -278 J/K at 25°C
Calculate ΔG° and state whether the equilibrium composition should favor reactants or products at standard conditions.
A)ΔG° = -332 kJ;the equilibrium composition should favor products.
B)ΔG° = -332 kJ;the equilibrium composition should favor reactants.
C)ΔG° = -166 kJ;the equilibrium composition should favor products.
D)ΔG° = -166 kJ;the equilibrium composition should favor reactants.
Calculate ΔG° and state whether the equilibrium composition should favor reactants or products at standard conditions.
A)ΔG° = -332 kJ;the equilibrium composition should favor products.
B)ΔG° = -332 kJ;the equilibrium composition should favor reactants.
C)ΔG° = -166 kJ;the equilibrium composition should favor products.
D)ΔG° = -166 kJ;the equilibrium composition should favor reactants.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
37
At 25°C,ΔH° = 1.895 kJ and ΔS° = -3.363 J/K for the transition C(graphite)→ C(diamond)
Based on these data,
A)graphite cannot be converted to diamond at 1 atm pressure.
B)diamond is more stable than graphite at all temperatures at 1 atm.
C)diamond is more stable than graphite below 290°C and graphite is more stable than diamond above 290°C.
D)graphite is more stable than diamond below 290°C and diamond is more stable than graphite above 290°C.
Based on these data,
A)graphite cannot be converted to diamond at 1 atm pressure.
B)diamond is more stable than graphite at all temperatures at 1 atm.
C)diamond is more stable than graphite below 290°C and graphite is more stable than diamond above 290°C.
D)graphite is more stable than diamond below 290°C and diamond is more stable than graphite above 290°C.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
38
For a spontaneous process
A)energy and entropy are conserved.
B)energy is conserved and the entropy of the system and surroundings increases.
C)the energy of the system and the surroundings decreases and the entropy of the system and surroundings increases.
D)both the energy and the entropy of the system and surroundings decrease.
A)energy and entropy are conserved.
B)energy is conserved and the entropy of the system and surroundings increases.
C)the energy of the system and the surroundings decreases and the entropy of the system and surroundings increases.
D)both the energy and the entropy of the system and surroundings decrease.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
39
According to the third law of thermodynamics,
A)energy is conserved in any transformation of matter.
B)the entropy increases for any spontaneous process.
C)the entropy of a perfectly ordered,crystalline substance is zero at 0 Kelvin.
D)the entropy of the universe increases for any spontaneous process.
A)energy is conserved in any transformation of matter.
B)the entropy increases for any spontaneous process.
C)the entropy of a perfectly ordered,crystalline substance is zero at 0 Kelvin.
D)the entropy of the universe increases for any spontaneous process.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
40
During perspiration,
A)the entropy of the water evaporated decreases and the entropy of the body decreases.
B)the entropy of the water evaporated decreases and the entropy of the body increases.
C)the entropy of the water evaporated increases and the entropy of the body decreases.
D)the entropy of the water evaporated increases and the entropy of the body increases.
A)the entropy of the water evaporated decreases and the entropy of the body decreases.
B)the entropy of the water evaporated decreases and the entropy of the body increases.
C)the entropy of the water evaporated increases and the entropy of the body decreases.
D)the entropy of the water evaporated increases and the entropy of the body increases.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
41
At high temperatures boron carbide vaporizes according to the equation B4C(s)⇌ 4 B(g)+ C(s)
Which equation describes the relationship between ΔG° and ΔG for this reaction?
A)ΔG = ΔG° + R T ln (pB ∙ [C]/[B4C])
B)ΔG = ΔG° + R T ln pB
C)ΔG = ΔG° + 4 R T ln pB
D)ΔG = ΔG° - 4 R T ln pB
Which equation describes the relationship between ΔG° and ΔG for this reaction?
A)ΔG = ΔG° + R T ln (pB ∙ [C]/[B4C])
B)ΔG = ΔG° + R T ln pB
C)ΔG = ΔG° + 4 R T ln pB
D)ΔG = ΔG° - 4 R T ln pB

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
42
At 25°C,ΔG°f is -620 kJ/mol for SiCl4(g)and -592 kJ/mol for MgCl2(s).Calculate ΔG° for the reaction,SiCl4(g)+ 2 Mg(s)→ 2 MgCl2(s)+ Si(s)and determine if the reaction is spontaneous at 25°C if the pressure of SiCl4(g)is 1 atm.
A)ΔG° = 28 kJ;the process is spontaneous.
B)ΔG° = 28 kJ;the process is nonspontaneous.
C)ΔG° = -564 kJ;the process is spontaneous.
D)ΔG° = -564 kJ;the process is nonspontaneous.
A)ΔG° = 28 kJ;the process is spontaneous.
B)ΔG° = 28 kJ;the process is nonspontaneous.
C)ΔG° = -564 kJ;the process is spontaneous.
D)ΔG° = -564 kJ;the process is nonspontaneous.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the standard free energy for the reaction given. 2 CH3OH(l)+ 3 O2(g)→ 2 CO2(g)+ 4 H2O(l) 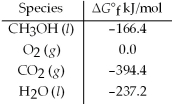
A)-465.2 kJ
B)-797.8 kJ
C)-1404.8 kJ
D)-2069.8 kJ

A)-465.2 kJ
B)-797.8 kJ
C)-1404.8 kJ
D)-2069.8 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction below ΔG° = +33.0 kJ,ΔH° = +92.2 kJ,and ΔS° = +198.7 J/K.Estimate the temperature at which this reaction becomes spontaneous. 2 NH3(g)→ N2(g)+ 3 H2(g)
A)0.464 K
B)166 K
C)298 K
D)464 K
A)0.464 K
B)166 K
C)298 K
D)464 K

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
45
For the evaporation of water during perspiration on a hot,dry day,
A)ΔH is positive and TΔS = ΔH.
B)ΔH is positive and TΔS > ΔH.
C)ΔH is positive and TΔS < ΔH.
D)ΔH is negative and TΔS is positive.
A)ΔH is positive and TΔS = ΔH.
B)ΔH is positive and TΔS > ΔH.
C)ΔH is positive and TΔS < ΔH.
D)ΔH is negative and TΔS is positive.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
46
For the reaction 3 C2H2(g)→ C6H6(l)at 25°C,the standard enthalpy change is -631 kJ and the standard entropy change is -430 J/K.Calculate the standard free energy change at 25°C?
A)-503 kJ
B)-10,120 kJ
C)619 kJ
D)-127 kJ
A)-503 kJ
B)-10,120 kJ
C)619 kJ
D)-127 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
47
For a reaction at constant temperature,as Q increases
A)ΔG and ΔG° increase.
B)ΔG and ΔG° decrease.
C)ΔG increases,but ΔG° remains constant.
D)ΔG decreases,but ΔG° remains constant.
A)ΔG and ΔG° increase.
B)ΔG and ΔG° decrease.
C)ΔG increases,but ΔG° remains constant.
D)ΔG decreases,but ΔG° remains constant.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following are unstable with respect to their constituent elements at 25°C? 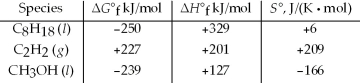
A)C8H18(l),CH3OH(l)
B)C8H18(l),C2H2(g)
C)C2H2(g)
D)CH3OH(l)

A)C8H18(l),CH3OH(l)
B)C8H18(l),C2H2(g)
C)C2H2(g)
D)CH3OH(l)

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is true?
A)As a reaction at constant temperature and pressure goes to equilibrium,|ΔG| decreases.
B)The larger ΔG°,the faster the reaction.
C)The standard state for solutes is the pure solute at 1 atm.
D)When a reaction reaches equilibrium,ΔG° = 0.
A)As a reaction at constant temperature and pressure goes to equilibrium,|ΔG| decreases.
B)The larger ΔG°,the faster the reaction.
C)The standard state for solutes is the pure solute at 1 atm.
D)When a reaction reaches equilibrium,ΔG° = 0.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
50
For any thermodynamic function Y,ΔY° for a reaction refers to the change in Y for the process in which
A)the mixed reactants at 1 atm go to equilibrium at 1 atm.
B)the separate reactants at 1 atm go to equilibrium at 1 atm.
C)the separate reactants in their standard states are completely converted to separate products in their standard states.
D)the spontaneous reaction occurs.
A)the mixed reactants at 1 atm go to equilibrium at 1 atm.
B)the separate reactants at 1 atm go to equilibrium at 1 atm.
C)the separate reactants in their standard states are completely converted to separate products in their standard states.
D)the spontaneous reaction occurs.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
51
The signs of ΔG,ΔH,and ΔS at 25°C are shown below for three reactions. reaction ΔG ΔH ΔS
I.- + +
II.- - +
III.- - -
Which reaction could go in the reverse direction at high temperature?
A)I
B)II
C)III
D)I and II
I.- + +
II.- - +
III.- - -
Which reaction could go in the reverse direction at high temperature?
A)I
B)II
C)III
D)I and II

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
52
A reaction has ΔH° = 61.9 kJ/mol and ΔS° = 405.4 J/K.Is this reaction spontaneous or nonspontaneous? At what temperature (if any)can the spontaneity be reversed?
A)nonspontaneous,can be made spontaneous at 153 K
B)nonspontaneous,cannot be made spontaneous at any temperature
C)spontaneous,can be made nonspontaneous at 153 K
D)spontaneous,cannot be made nonspontaneous at any temperature
A)nonspontaneous,can be made spontaneous at 153 K
B)nonspontaneous,cannot be made spontaneous at any temperature
C)spontaneous,can be made nonspontaneous at 153 K
D)spontaneous,cannot be made nonspontaneous at any temperature

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
53
At 25°C,ΔG° = -198 kJ for the reaction,NO(g)+ O3(g)⇌ NO2(g)+ O2(g). Calculate ΔG under the following conditions: 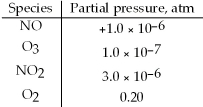
A)-159 kJ
B)-167 kJ
C)-198 kJ
D)-236 kJ

A)-159 kJ
B)-167 kJ
C)-198 kJ
D)-236 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
54
A positive value of ΔG°f for a solid compound at 25°C means the
A)compound cannot exist at 25°C and 1 atm.
B)compound must be a liquid or a gas at 25°C and 1 atm.
C)process of forming the compound from the elements is exothermic.
D)process of forming the compound from the stable elements at 25°C and 1 atm is nonspontaneous.
A)compound cannot exist at 25°C and 1 atm.
B)compound must be a liquid or a gas at 25°C and 1 atm.
C)process of forming the compound from the elements is exothermic.
D)process of forming the compound from the stable elements at 25°C and 1 atm is nonspontaneous.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the standard free energy change at 25°C for the reaction 2 NO(g)+ O2(g)→ 2 NO2(g). 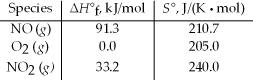
A)-4.7 kJ
B)-72.6 kJ
C)-157.8 kJ
D)-532.6 kJ

A)-4.7 kJ
B)-72.6 kJ
C)-157.8 kJ
D)-532.6 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
56
At 2600 K,ΔG° = 775 kJ for the vaporization of boron carbide: B4C(s)⇌ 4 B(g)+ C(s)
Find ΔG and determine if the process is spontaneous if the reaction vessel contains 4.00 mol B4C(s),0.400 mol of C(s),and B(g)at a partial pressure of 1.0 × 10-5 atm.At this temperature,R T = 21.6 kJ.
A)ΔG = -270 kJ;spontaneous.
B)ΔG = -270 kJ;nonspontaneous.
C)ΔG = -220 kJ;spontaneous.
D)ΔG = -220 kJ;nonspontaneous.
Find ΔG and determine if the process is spontaneous if the reaction vessel contains 4.00 mol B4C(s),0.400 mol of C(s),and B(g)at a partial pressure of 1.0 × 10-5 atm.At this temperature,R T = 21.6 kJ.
A)ΔG = -270 kJ;spontaneous.
B)ΔG = -270 kJ;nonspontaneous.
C)ΔG = -220 kJ;spontaneous.
D)ΔG = -220 kJ;nonspontaneous.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
57
A reaction has ΔH° = -60.9 kJ/mol and ΔS° = 266.6 J/K.Is this reaction spontaneous or nonspontaneous? At what temperature (if any)can the spontaneity be reversed?
A)nonspontaneous,can be made spontaneous at 228 K
B)nonspontaneous,cannot be made spontaneous at any temperature
C)spontaneous,can be made nonspontaneous at 228 K
D)spontaneous,cannot be made nonspontaneous at any temperature
A)nonspontaneous,can be made spontaneous at 228 K
B)nonspontaneous,cannot be made spontaneous at any temperature
C)spontaneous,can be made nonspontaneous at 228 K
D)spontaneous,cannot be made nonspontaneous at any temperature

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
58
Which is the lowest at 25°C?
A)ΔG°f for H2O (s)
B)ΔG°f for H2O (l)
C)ΔG°f for H2O (g)
D)1/2ΔG°f for O2 (g)plus ΔG°f for H2O (g)
A)ΔG°f for H2O (s)
B)ΔG°f for H2O (l)
C)ΔG°f for H2O (g)
D)1/2ΔG°f for O2 (g)plus ΔG°f for H2O (g)

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
59
Water can be made from elemental hydrogen and oxygen.Which equation can be used to determine ΔGform from its elemental parts?
A)ΔGform = Δ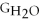
- Δ
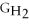
- Δ
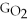
B)ΔGform = 2Δ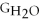
- 2Δ
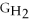
- Δ
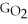
C)ΔGform = Δ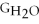
- 2ΔGH - ΔGO
D)ΔGform = 2Δ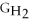
+ Δ
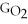
- 2Δ
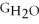
A)ΔGform = Δ
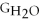
- Δ
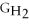
- Δ
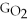
B)ΔGform = 2Δ
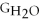
- 2Δ
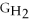
- Δ
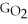
C)ΔGform = Δ
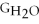
- 2ΔGH - ΔGO
D)ΔGform = 2Δ
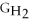
+ Δ
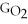
- 2Δ
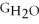

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
60
Which equation can be used to determine ΔGform for C2H5OH from its elemental parts.Be sure that the reaction is balanced. 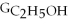
A)ΔGform = Δ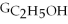
- ΔGC - Δ
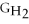
- Δ
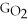
B)ΔGform = 2Δ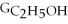
- 4ΔGC - 6Δ
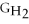
- Δ
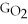
C)ΔGform = 2ΔGC + 6ΔGH + ΔGO
D)ΔGform = Δ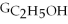
- 2ΔGC - 3Δ
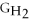
- Δ
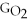
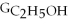
A)ΔGform = Δ
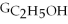
- ΔGC - Δ
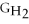
- Δ
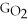
B)ΔGform = 2Δ
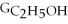
- 4ΔGC - 6Δ
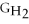
- Δ
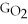
C)ΔGform = 2ΔGC + 6ΔGH + ΔGO
D)ΔGform = Δ
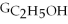
- 2ΔGC - 3Δ
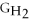
- Δ
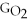

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
61
If ΔG° is negative for a reaction,
A)K < 0.
B)K = 0.
C)K is between 0 and 1.
D)K > 1.
A)K < 0.
B)K = 0.
C)K is between 0 and 1.
D)K > 1.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
62
An ideal gas is expanded at constant temperature.What are the signs (+,-,or 0)of ΔH,ΔS,and ΔG for this system? 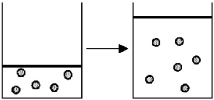
A)ΔH = +,ΔS = -,ΔG = +
B)ΔH = 0,ΔS = +,ΔG = -
C)ΔH = 0,ΔS = -,ΔG = +
D)ΔH = -,ΔS = +,ΔG = -

A)ΔH = +,ΔS = -,ΔG = +
B)ΔH = 0,ΔS = +,ΔG = -
C)ΔH = 0,ΔS = -,ΔG = +
D)ΔH = -,ΔS = +,ΔG = -

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
63
What is the relationship between ΔG and the ΔG°F for the reaction below? MgF2(s)→ Mg2+(aq)+ 2 F-(aq)
A)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)] - ΔG°f [MgF2 (s)]} + RT ln ([Mg2+] [F-]2/[MgF2])
B)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)] - ΔG°f [MgF2 (s)]} + RT ln ([Mg2+] [F-])2)
C)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)]} + RT ln ([Mg2+] [F-]2)
D)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)] - ΔG°f [MgF2 (s)]} + RT ln Ksp
A)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)] - ΔG°f [MgF2 (s)]} + RT ln ([Mg2+] [F-]2/[MgF2])
B)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)] - ΔG°f [MgF2 (s)]} + RT ln ([Mg2+] [F-])2)
C)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)]} + RT ln ([Mg2+] [F-]2)
D)ΔG = {ΔG°f [Mg2+ (aq)] + 2 ΔG°f [F- (aq)] - ΔG°f [MgF2 (s)]} + RT ln Ksp

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
64
ΔG = ΔG° for a reaction
A)if Q = K.
B)if Q = 1.
C)at STP.
D)at the start of the reaction.
A)if Q = K.
B)if Q = 1.
C)at STP.
D)at the start of the reaction.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
65
If ΔG is small and positive,
A)the forward reaction is spontaneous and the system is far from equilibrium.
B)the forward reaction is spontaneous and the system is near equilibrium.
C)the reverse reaction is spontaneous and the system is far from equilibrium.
D)the reverse reaction is spontaneous and the system is near equilibrium.
A)the forward reaction is spontaneous and the system is far from equilibrium.
B)the forward reaction is spontaneous and the system is near equilibrium.
C)the reverse reaction is spontaneous and the system is far from equilibrium.
D)the reverse reaction is spontaneous and the system is near equilibrium.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate Ksp for PbI2 at 25°C based on the following data: 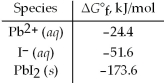
A)4 × 10-31
B)8 × 10-18
C)9 × 10-9
D)5 × 10-5

A)4 × 10-31
B)8 × 10-18
C)9 × 10-9
D)5 × 10-5

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
67
For the following reaction find Kp at 25°C and indicate whether Kp should increase or decrease as the temperature rises. NH4HS(s)⇌ H2S(g)+ NH3(g)ΔH° = 83.47 kJ and ΔG° = 17.5 kJ at 25°C.
A)Kp = 8.6 × 10-4 and Kp should increase as the temperature rises.
B)Kp = 8.6 × 10-4 and Kp should decrease as the temperature rises.
C)Kp = 1.2 × 103 and Kp should increase as the temperature rises.
D)Kp = 1.2 × 103 and Kp should decrease as the temperature rises.
A)Kp = 8.6 × 10-4 and Kp should increase as the temperature rises.
B)Kp = 8.6 × 10-4 and Kp should decrease as the temperature rises.
C)Kp = 1.2 × 103 and Kp should increase as the temperature rises.
D)Kp = 1.2 × 103 and Kp should decrease as the temperature rises.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
68
If Q increases,
A)ΔG increases and the reaction becomes more spontaneous.
B)ΔG increases and the reaction becomes less spontaneous.
C)ΔG decreases and the reaction becomes more spontaneous.
D)ΔG decreases and the reaction becomes less spontaneous.
A)ΔG increases and the reaction becomes more spontaneous.
B)ΔG increases and the reaction becomes less spontaneous.
C)ΔG decreases and the reaction becomes more spontaneous.
D)ΔG decreases and the reaction becomes less spontaneous.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following must be true in order for this reaction to always proceed spontaneously?
A)K = Q
B)K > Q
C)K < Q
D)None of these,spontaneity is dependent on reaction conditions.
A)K = Q
B)K > Q
C)K < Q
D)None of these,spontaneity is dependent on reaction conditions.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
70
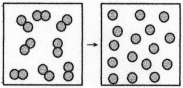
The figure above represents the nonspontaneous reaction O2(g)→ 2O(g).What are the signs (+ or -)of ΔH,ΔS,and ΔG for this process?
A)ΔH = +,ΔS = +,ΔG = +
B)ΔH = +,ΔS = +,ΔG = -
C)ΔH = -,ΔS = -,ΔG = +
D)ΔH = -,ΔS = -,ΔG = -

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
71
At high temperatures,boron carbide vaporizes according to B4C(s)⇌ 4 B(g)+ C(s)
At 2500 K,the equilibrium pressure of B(g)is 0.0342 mm Hg over a mixture of 0.300 mol B4C(s)and 0.500 mol C(s).Calculate ΔG° for this process.
A)832 kJ
B)799 kJ
C)281 kJ
D)247 kJ
At 2500 K,the equilibrium pressure of B(g)is 0.0342 mm Hg over a mixture of 0.300 mol B4C(s)and 0.500 mol C(s).Calculate ΔG° for this process.
A)832 kJ
B)799 kJ
C)281 kJ
D)247 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
72
Solid NaHCO3 is heated to 90°C.At equilibrium the total pressure of the gases produced is 0.545 atm.Calculate ΔG° at 90°C for the reaction 2 NaHCO3(s)⇌ Na2CO3(s)+ H2O(g)+ CO2(g).
A)-7.85 kJ
B)-3.67 kJ
C)+3.67 kJ
D)+7.85 kJ
A)-7.85 kJ
B)-3.67 kJ
C)+3.67 kJ
D)+7.85 kJ

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
73
The figure represents the spontaneous deposition of iodine in which iodine vapor,I2(g),becomes crystalline iodine solid I2(s): I2(g): → I2(s).What are the signs (+ or -)of ΔH,ΔS,and ΔG for this process? 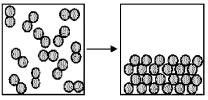
A)ΔH = +,ΔS = +,ΔG = +
B)ΔH = +,ΔS = +,ΔG = -
C)ΔH = -,ΔS = -,ΔG = +
D)ΔH = -,ΔS = -,ΔG = -

A)ΔH = +,ΔS = +,ΔG = +
B)ΔH = +,ΔS = +,ΔG = -
C)ΔH = -,ΔS = -,ΔG = +
D)ΔH = -,ΔS = -,ΔG = -

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
74
If ΔG° is positive for a reaction,
A)K < 0.
B)K = 0.
C)K is between 0 and 1.
D)K > 1.
A)K < 0.
B)K = 0.
C)K is between 0 and 1.
D)K > 1.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
75
The figure represents the spontaneous evaporation of nitrogen in which liquid nitrogen,N2(l),becomes gaseous nitrogen,N2(g): N2(l)→ N2(g).What are the signs (+ or -)of ΔH,ΔS,and ΔG for this process? 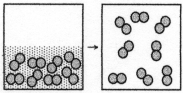
A)ΔH = +,ΔS = +,ΔG = +
B)ΔH = +,ΔS = +,ΔG = -
C)ΔH = -,ΔS = -,ΔG = +
D)ΔH = -,ΔS = -,ΔG = -

A)ΔH = +,ΔS = +,ΔG = +
B)ΔH = +,ΔS = +,ΔG = -
C)ΔH = -,ΔS = -,ΔG = +
D)ΔH = -,ΔS = -,ΔG = -

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
76
What is the relationship between ΔG,Qp,and Kp for a reaction involving gases?
A)ΔG = Qp/Kp
B)ΔG = Kp/Qp
C)ΔG = RTln(Qp/Kp)
D)ΔG = RTln(Kp/Qp)
A)ΔG = Qp/Kp
B)ΔG = Kp/Qp
C)ΔG = RTln(Qp/Kp)
D)ΔG = RTln(Kp/Qp)

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
77
In figure (1)below argon atoms,represented by unshaded spheres,and neon atoms,represented by shaded spheres,are in separate compartments.Figure (2)shows the equilibrium state of the system after the stopcock separating the two compartments is opened.Assuming that argon and neon behave as ideal gases,what are the signs (+,-,or 0)of ΔH,ΔS,and ΔG for this process? 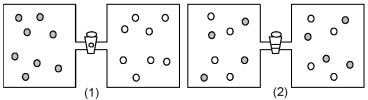
A)ΔH = +,ΔS = -,ΔG = +
B)ΔH = 0,ΔS = +,ΔG = -
C)ΔH = 0,ΔS = -,ΔG = +
D)ΔH = -,ΔS = +,ΔG = -

A)ΔH = +,ΔS = -,ΔG = +
B)ΔH = 0,ΔS = +,ΔG = -
C)ΔH = 0,ΔS = -,ΔG = +
D)ΔH = -,ΔS = +,ΔG = -

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
78

The figure above represents the reaction O2(g)→ 2O(g),which is nonspontaneous at 25°C.How will the spontaneity of this reaction vary with temperature? This reaction is
A)nonspontaneous at all temperatures.
B)nonspontaneous at high temperatures and spontaneous at low temperatures.
C)spontaneous at high temperatures and nonspontaneous at low temperatures.
D)spontaneous at all temperatures.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
79
When equilibrium is reached at constant temperature and pressure,
A)Q = 1.
B)ΔG° = 0.
C)S is maximized.
D)G is minimized.
A)Q = 1.
B)ΔG° = 0.
C)S is maximized.
D)G is minimized.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
80
In figure (1)below oxygen molecules,represented by unshaded spheres,and chlorine molecules,represented by shaded spheres,are in separate compartments.Figure (2)shows the equilibrium state of the system after the stopcock separating the two compartments is opened.Assuming the oxygen and the chlorine behave as ideal gases,what are the signs (+,-,or 0)of ΔH,ΔS,and ΔG for this process? 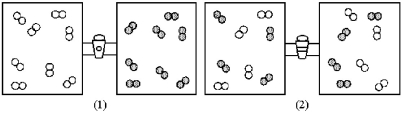
A)ΔH = +,ΔS = -,ΔG = +
B)ΔH = 0,ΔS = +,ΔG = -
C)ΔH = 0,ΔS = -,ΔG = +
D)ΔH = -,ΔS = +,ΔG = -

A)ΔH = +,ΔS = -,ΔG = +
B)ΔH = 0,ΔS = +,ΔG = -
C)ΔH = 0,ΔS = -,ΔG = +
D)ΔH = -,ΔS = +,ΔG = -

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck


