Deck 2: Atoms, molecules, and Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/275
Play
Full screen (f)
Deck 2: Atoms, molecules, and Ions
1
According to history,the concept that all matter is composed of atoms was first proposed by
A)the Greek philosopher Democritus,but not widely accepted until modern times.
B)Dalton,but not widely accepted until the work of Mendeleev.
C)Dalton,but not widely accepted until the work of Einstein.
D)Dalton,and widely accepted within a few decades.
A)the Greek philosopher Democritus,but not widely accepted until modern times.
B)Dalton,but not widely accepted until the work of Mendeleev.
C)Dalton,but not widely accepted until the work of Einstein.
D)Dalton,and widely accepted within a few decades.
the Greek philosopher Democritus,but not widely accepted until modern times.
2
Sodium metal and water react to form hydrogen and sodium hydroxide.If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide,what mass of water was consumed in the reaction?
A)4.68 g
B)5.98 g
C)10.14 g
D)10.66 g
A)4.68 g
B)5.98 g
C)10.14 g
D)10.66 g
4.68 g
3
Elements in a periodic group have similar
A)chemical properties.
B)densities.
C)masses.
D)physical properties.
A)chemical properties.
B)densities.
C)masses.
D)physical properties.
chemical properties.
4
Gaseous elements characterized by low reactivity are found in group ________ of the periodic table.
A)5A
B)6A
C)7A
D)8A
A)5A
B)6A
C)7A
D)8A

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
5
Which is not true?
A)Mendeleev ended each row in his periodic table with an inert gas.
B)Mendeleev left gaps in his periodic table for undiscovered elements.
C)Mendeleev ordered the elements in his periodic table by atomic weight.
D)Mendeleev's periodic table predated the concept of electron configuration.
A)Mendeleev ended each row in his periodic table with an inert gas.
B)Mendeleev left gaps in his periodic table for undiscovered elements.
C)Mendeleev ordered the elements in his periodic table by atomic weight.
D)Mendeleev's periodic table predated the concept of electron configuration.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
6
Which element has the chemical symbol,P?
A)lead
B)phosphorus
C)platinum
D)potassium
A)lead
B)phosphorus
C)platinum
D)potassium

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of pure lithium carbonate contains 18.8% lithium by mass.What is the % lithium by mass in a sample of pure lithium carbonate that has twice the mass of the first sample?
A)9.40%
B)18.8%
C)37.6%
D)75.2%
A)9.40%
B)18.8%
C)37.6%
D)75.2%

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
8
Mendeleev arranged the elements according to
A)atomic number and atomic weight.
B)atomic weight and chemical reactivity.
C)electron configuration and atomic weight.
D)physical state and relative abundance.
A)atomic number and atomic weight.
B)atomic weight and chemical reactivity.
C)electron configuration and atomic weight.
D)physical state and relative abundance.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
9
The observation that 4.0 g of hydrogen reacts with 32.0 g of oxygen to form a product with O:H mass ratio = 8:1,and 6.0 g of hydrogen reacts with 48.0 g of oxygen to form the same product with O/H mass ratio = 8:1 is evidence for the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
10
Most elements in the periodic table are
A)metals.
B)non-metals.
C)noble gases.
D)semi-metals.
A)metals.
B)non-metals.
C)noble gases.
D)semi-metals.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
11
The observation that 15.0 g of hydrogen reacts with 120.0 g of oxygen to form 135.0 g of water is evidence for the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
12
The vertical columns of the periodic table are called
A)groups.
B)periods.
C)triads.
D)elements.
A)groups.
B)periods.
C)triads.
D)elements.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
13
Methane and oxygen react to form carbon dioxide and water.What mass of water is formed if 3.2 g of methane reacts with 12.8 g of oxygen to produce 8.8 g of carbon dioxide?
A)7.2 g
B)8.8 g
C)14.8 g
D)16.0 g
A)7.2 g
B)8.8 g
C)14.8 g
D)16.0 g

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
14
What is the chemical symbol for manganese?
A)Hg
B)Mg
C)Mn
D)Na
A)Hg
B)Mg
C)Mn
D)Na

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements does not describe a physical property of chlorine?
A)Chlorine combines with sodium to form table salt.
B)The color of chorine gas is green.
C)The density of chlorine gas at standard temperature and pressure is 3.17 g/L.
D)The freezing point of chlorine is -101°C.
A)Chlorine combines with sodium to form table salt.
B)The color of chorine gas is green.
C)The density of chlorine gas at standard temperature and pressure is 3.17 g/L.
D)The freezing point of chlorine is -101°C.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
16
Which horizontal row of the periodic table contains the most elements?
A)row 4
B)row 5
C)row 6
D)They all contain the same number of elements.
A)row 4
B)row 5
C)row 6
D)They all contain the same number of elements.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
17
Which group of elements reacts violently with water?
A)halogens
B)noble gases
C)alkali metals
D)alkaline earth metals
A)halogens
B)noble gases
C)alkali metals
D)alkaline earth metals

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements does not describe a chemical property of oxygen?
A)Iron will rust in the presence of oxygen.
B)Oxygen combines with carbon to form carbon dioxide gas.
C)The pressure is caused by collision of oxygen molecules with the sides of a container.
D)When coal is burned in oxygen,the process is called combustion.
A)Iron will rust in the presence of oxygen.
B)Oxygen combines with carbon to form carbon dioxide gas.
C)The pressure is caused by collision of oxygen molecules with the sides of a container.
D)When coal is burned in oxygen,the process is called combustion.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
19
Which group 5A element is most metallic?
A)N
B)P
C)Sb
D)Bi
A)N
B)P
C)Sb
D)Bi

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
20
The horizontal rows of the periodic table are called
A)groups.
B)periods.
C)triads.
D)elements.
A)groups.
B)periods.
C)triads.
D)elements.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following represent isotopes? A: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_c81a_a2f7_1374164ca05e_TB4940_11.jpg) [ ] B:
[ ] B: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_c81b_a2f7_291dd3ab4657_TB4940_11.jpg) [ ] C:
[ ] C: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_c81c_a2f7_ed756eacd689_TB4940_11.jpg) [ ] D:
[ ] D: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_ef2d_a2f7_53a7d3d285d6_TB4940_11.jpg) [ ]
[ ]
A)A and B
B)A and C
C)A and D
D)C and D
![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_c81a_a2f7_1374164ca05e_TB4940_11.jpg) [ ] B:
[ ] B: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_c81b_a2f7_291dd3ab4657_TB4940_11.jpg) [ ] C:
[ ] C: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_c81c_a2f7_ed756eacd689_TB4940_11.jpg) [ ] D:
[ ] D: ![<strong>Which of the following represent isotopes? A: [ ] B: [ ] C: [ ] D: [ ]</strong> A)A and B B)A and C C)A and D D)C and D](https://storage.examlex.com/TB4940/11ea7e2d_d089_ef2d_a2f7_53a7d3d285d6_TB4940_11.jpg) [ ]
[ ]A)A and B
B)A and C
C)A and D
D)C and D

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following two atoms are isotopes?
A)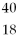 Ar and
Ar and 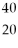 Ca
Ca
B)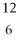 C and
C and 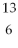 C
C
C)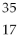 l and
l and 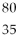 Br
Br
D)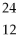 Mg and
Mg and 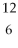 C
C
A)
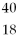 Ar and
Ar and 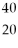 Ca
CaB)
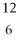 C and
C and 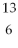 C
CC)
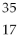 l and
l and 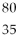 Br
BrD)
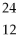 Mg and
Mg and 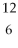 C
C
Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
23
The mass number of an atom is equal to the number of
A)electrons.
B)neutrons.
C)protons.
D)protons plus neutrons.
A)electrons.
B)neutrons.
C)protons.
D)protons plus neutrons.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
24
The charge-to-mass ratio of an electron was established by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
25
Boron-9 can be represented as
A)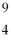 Be.
Be.
B)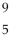 B.
B.
C)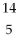 B.
B.
D)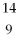 B.
B.
A)
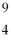 Be.
Be.B)
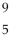 B.
B.C)
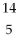 B.
B.D)
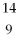 B.
B.
Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
26
Which subatomic particle has the smallest mass?
A)a proton
B)a neutron
C)an electron
D)an alpha particle
A)a proton
B)a neutron
C)an electron
D)an alpha particle

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
27
How many electrons are in a neutral atom of iodine-131?
A)1
B)53
C)54
D)131
A)1
B)53
C)54
D)131

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a part of Dalton's atomic theory?
A)Atoms are rearranged but not changed during a chemical reaction.
B)Atoms break down during radioactive decay.
C)Atoms contain protons,neutrons,and electrons.
D)Isotopes of the same element have different masses.
A)Atoms are rearranged but not changed during a chemical reaction.
B)Atoms break down during radioactive decay.
C)Atoms contain protons,neutrons,and electrons.
D)Isotopes of the same element have different masses.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
29
What is the chemical symbol for an atom that has 29 protons and 36 neutrons?
A)Cu
B)Kr
C)N
D)Tb
A)Cu
B)Kr
C)N
D)Tb

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
30
The existence of neutrons in the nucleus of an atom was demonstrated by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
31
The observation that hydrogen and oxygen can react to form two compounds with different chemical and physical properties,one having an O:H mass ratio = 8:1 and the other having an O:H mass ratio = 16:1 is consistent with the law of
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.
A)definite proportions.
B)energy conservation.
C)mass conservation.
D)multiple proportions.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements is not a postulate of Dalton's atomic theory?
A)Each element is characterized by the mass of its atoms.
B)Atoms are composed of protons,neutrons,and electrons.
C)Chemical reactions only rearrange atomic combinations.
D)Elements are composed of atoms.
A)Each element is characterized by the mass of its atoms.
B)Atoms are composed of protons,neutrons,and electrons.
C)Chemical reactions only rearrange atomic combinations.
D)Elements are composed of atoms.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
33
How many protons (p)and neutrons (n)are in an atom of 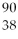 Sr?
Sr?
A)38 p,52 n
B)38 p,90 n
C)52 p,38 n
D)90 p,38 n
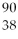 Sr?
Sr?A)38 p,52 n
B)38 p,90 n
C)52 p,38 n
D)90 p,38 n

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
34
How many protons (p)and neutrons (n)are in an atom of calcium-46?
A)20 p,26 n
B)20 p,46 n
C)26 p,20 n
D)46 p,60 n
A)20 p,26 n
B)20 p,46 n
C)26 p,20 n
D)46 p,60 n

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
35
The symbol that is usually used to represent atomic number is
A)A.
B)N.
C)X.
D)Z.
A)A.
B)N.
C)X.
D)Z.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is not explained by Dalton's atomic theory?
A)conservation of mass during a chemical reaction
B)the existence of more than one isotope of an element
C)the law of definite proportions
D)the law of multiple proportions
A)conservation of mass during a chemical reaction
B)the existence of more than one isotope of an element
C)the law of definite proportions
D)the law of multiple proportions

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
37
The current model of the atom in which essentially all of an atom's mass is contained in a very small nucleus,whereas most of an atom's volume is due to the space in which the atom's electrons move was established by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of pure calcium fluoride with a mass of 15.0 g contains 7.70 g of calcium.How much calcium is contained in 45.0 g of calcium fluoride?
A)2.56 g
B)7.70 g
C)15.0 g
D)23.1 g
A)2.56 g
B)7.70 g
C)15.0 g
D)23.1 g

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
39
The existence of electrons in atoms of all elements was demonstrated by
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these
A)Millikan's oil drop experiment.
B)Rutherford's gold foil experiment.
C)Thomson's cathode ray tube experiment.
D)None of these

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
40
Most of the alpha particles directed at a thin gold foil in Rutherford's experiment
A)bounced directly back from the foil.
B)passed directly through the foil undeflected.
C)passed through the foil but were deflected at an angle.
D)were absorbed by the foil.
A)bounced directly back from the foil.
B)passed directly through the foil undeflected.
C)passed through the foil but were deflected at an angle.
D)were absorbed by the foil.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
41
What is the mass of one atom of the element hydrogen?
A)2.0 g
B)1.0 g
C)3.4 × 10-24 g
D)1.7 × 10-24 g
A)2.0 g
B)1.0 g
C)3.4 × 10-24 g
D)1.7 × 10-24 g

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
42
How many electrons are in the ion,CO32-?
A)16
B)28
C)30
D)32
A)16
B)28
C)30
D)32

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the species below has 28 protons and 26 electrons?
A)Fe2+
B)Ni2+
C)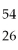
Fe
D)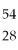
Ni
A)Fe2+
B)Ni2+
C)
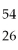
Fe
D)
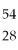
Ni

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
44
How many moles and how many atoms of zinc are in a sample weighing 34.9 g?
A)0.533 mol,8.85 ×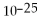
Atoms
B)0.533 mol,3.21 ×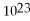
Atoms
C)1.87 mol,3.10 ×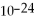
Atoms
D)1.87 mol,1.13 ×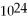
Atoms
A)0.533 mol,8.85 ×
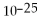
Atoms
B)0.533 mol,3.21 ×
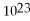
Atoms
C)1.87 mol,3.10 ×
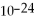
Atoms
D)1.87 mol,1.13 ×
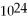
Atoms

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
45
What is the identity of element Q if the ion Q2+ contains 10 electrons?
A)C
B)O
C)Ne
D)Mg
A)C
B)O
C)Ne
D)Mg

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
46
How many electrons are in the ion,Zn2+?
A)28
B)30
C)32
D)65
A)28
B)30
C)32
D)65

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
47
The element antimony has an atomic weight of 121.757 amu and only two naturally-occurring isotopes.One isotope has an abundance of 57.3% and an isotopic mass of 120.904 amu.Based on these data,what is the mass of the other isotope?
A)121.757 amu
B)122.393 amu
C)122.610 amu
D)122.902 amu
A)121.757 amu
B)122.393 amu
C)122.610 amu
D)122.902 amu

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
48
Three atoms have the following properties. 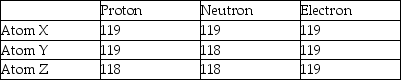 The elements Y and Z are best described as
The elements Y and Z are best described as
A)isotopes.
B)cations.
C)different elements.
D)anions.
 The elements Y and Z are best described as
The elements Y and Z are best described asA)isotopes.
B)cations.
C)different elements.
D)anions.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
49
The smallest sample of carbon atoms that can be observed with the naked eye has a mass of approximately 2 × 10-8 g.Given that 1 g = 6.02 × 1023 amu,and that carbon has an atomic weight of 12.01 amu,determine the number of carbon atoms present in the sample.
A)1 × 1015
B)1 × 1016
C)1 × 1017
D)6 × 1023
A)1 × 1015
B)1 × 1016
C)1 × 1017
D)6 × 1023

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
50
Three atoms have the following properties. 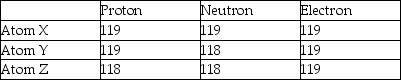 The elements X and Y are best described as
The elements X and Y are best described as
A)isotopes.
B)cations.
C)different elements.
D)anions.
 The elements X and Y are best described as
The elements X and Y are best described asA)isotopes.
B)cations.
C)different elements.
D)anions.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
51
Three atoms have the following properties. 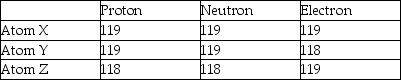 Which of the following statements is true?
Which of the following statements is true?
A)Element Y and Z are isotopes of X.
B)Element Y is an isotope of Z.
C)Element Y is an ion of X.
D)Element Z is an ion of Y.
 Which of the following statements is true?
Which of the following statements is true?A)Element Y and Z are isotopes of X.
B)Element Y is an isotope of Z.
C)Element Y is an ion of X.
D)Element Z is an ion of Y.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
52
Butyric acid has the structural formula given below. 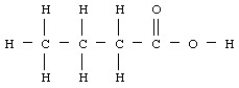 What is the molecular or chemical formula for butyric acid?
What is the molecular or chemical formula for butyric acid?
A)CHO
B)C2H4O
C)C4H8O2
D)C5H8O3
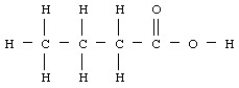 What is the molecular or chemical formula for butyric acid?
What is the molecular or chemical formula for butyric acid?A)CHO
B)C2H4O
C)C4H8O2
D)C5H8O3

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
53
How many electrons are in the ion,P3-?
A)12
B)18
C)28
D)34
A)12
B)18
C)28
D)34

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
54
What is the identity of the element with 6 protons,7 neutrons,and 6 electrons?
A)C
B)N
C)Al
D)Mg
A)C
B)N
C)Al
D)Mg

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
55
An element has two naturally occurring isotopes.One has an abundance of 37.4% and an isotopic mass of 184.953 amu,and the other has an abundance of 62.6% and a mass of 186.956 amu.What is the atomic weight of the element?
A)185.702 amu
B)185.954 amu
C)186.207 amu
D)186.956 amu
A)185.702 amu
B)185.954 amu
C)186.207 amu
D)186.956 amu

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
56
What type of bonding is found in the compound PCl5?
A)covalent bonding
B)hydrogen bonding
C)ionic bonding
D)metallic bonding
A)covalent bonding
B)hydrogen bonding
C)ionic bonding
D)metallic bonding

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
57
How many protons (p),neutrons (n),and electrons (e)are in one atom of 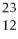 Mg?
Mg?
A)12 p,12 n,12 e
B)12 p,11 n,12 e
C)12 p,11 n,10 e
D)12 p,11 n,14 e
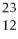 Mg?
Mg?A)12 p,12 n,12 e
B)12 p,11 n,12 e
C)12 p,11 n,10 e
D)12 p,11 n,14 e

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
58
The atoms of a particular element all have the same number of protons as neutrons.Which of the following must be true?
A)The atomic weight must be a whole number.
B)The mass number for each atom must equal the atomic weight of the element.
C)The mass number must be exactly twice the atomic number for each atom.
D)All of these are true.
A)The atomic weight must be a whole number.
B)The mass number for each atom must equal the atomic weight of the element.
C)The mass number must be exactly twice the atomic number for each atom.
D)All of these are true.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
59
Steel is galvanized by giving it a surface coating of zinc.Galvanized steel is an example of
A)a compound.
B)an element.
C)a mixture.
D)an ion.
A)a compound.
B)an element.
C)a mixture.
D)an ion.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the chemical symbol of element Q in 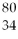 Q.
Q.
A)Br
B)Hg
C)Pd
D)Se
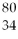 Q.
Q.A)Br
B)Hg
C)Pd
D)Se

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
61
The formula for dinitrogen trioxide is
A)N(OH)3.
B)(NO3)2.
C)N2O3.
D)N3O2.
A)N(OH)3.
B)(NO3)2.
C)N2O3.
D)N3O2.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
62
What is the name of the compound formed between Ca and N?
A)calcium dinitride
B)calcium trinitride
C)monocalcium trinitride
D)calcium nitride
A)calcium dinitride
B)calcium trinitride
C)monocalcium trinitride
D)calcium nitride

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
63
By analogy with the oxoanions of sulfur,H2TeO3 would be named
A)hydrotellurous acid.
B)pertelluric acid.
C)telluric acid.
D)tellurous acid.
A)hydrotellurous acid.
B)pertelluric acid.
C)telluric acid.
D)tellurous acid.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
64
The ions ClO4-,ClO3-,ClO2-,and ClO- are named respectively
A)hypochlorate,chlorate,chlorite,perchlorite.
B)hypochlorite,chlorite,chlorate,perchlorate.
C)perchlorate,chlorate,chlorite,hypochlorite.
D)perchlorite,chlorite,chlorate,hypochlorate.
A)hypochlorate,chlorate,chlorite,perchlorite.
B)hypochlorite,chlorite,chlorate,perchlorate.
C)perchlorate,chlorate,chlorite,hypochlorite.
D)perchlorite,chlorite,chlorate,hypochlorate.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
65
The solid compound,Na2CO3,contains
A)Na+,C4+,and O2- ions.
B)Na+ ions and CO32-ions.
C)Na2+ and CO32- ions.
D)Na2CO3 molecules.
A)Na+,C4+,and O2- ions.
B)Na+ ions and CO32-ions.
C)Na2+ and CO32- ions.
D)Na2CO3 molecules.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
66
How many of the following names are correct? 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
67
What group of elements does the shaded area in the following periodic table indicate? 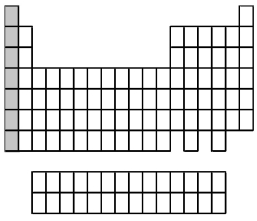
A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
68
The definitive distinction between ionic bonding and covalent bonding is that
A)ionic bonding involves a sharing of electrons and covalent bonding involves a transfer of electrons.
B)ionic bonding involves a transfer of electrons and covalent bonding involves a sharing of electrons.
C)ionic bonding requires two nonmetals and covalent bonding requires a metal and a nonmetal.
D)covalent bonding requires two nonmetals and ionic bonding requires a metal and a nonmetal.
A)ionic bonding involves a sharing of electrons and covalent bonding involves a transfer of electrons.
B)ionic bonding involves a transfer of electrons and covalent bonding involves a sharing of electrons.
C)ionic bonding requires two nonmetals and covalent bonding requires a metal and a nonmetal.
D)covalent bonding requires two nonmetals and ionic bonding requires a metal and a nonmetal.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
69
The compound,NO2,is named
A)nitrate.
B)nitrite.
C)nitrogen dioxide.
D)nitrogen(IV)oxide.
A)nitrate.
B)nitrite.
C)nitrogen dioxide.
D)nitrogen(IV)oxide.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
70
The thiosulfate ion is
A)HS-.
B)HSO42-.
C)SO52-.
D)S2O32-.
A)HS-.
B)HSO42-.
C)SO52-.
D)S2O32-.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
71
What group of elements does the shaded area in the following periodic table indicate? 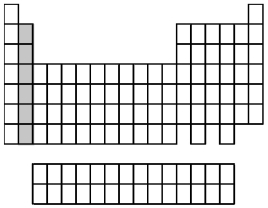
A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
72
What group of elements does the shaded area in the following periodic table indicate? 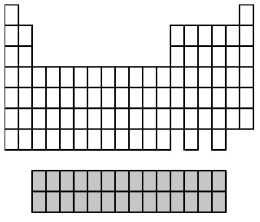
A)alkali metals
B)alkaline earth metals
C)inner transition metals
D)transition metals

A)alkali metals
B)alkaline earth metals
C)inner transition metals
D)transition metals

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following statements concerning ionic compounds is true?
A)Essentially all ionic compounds are solids at room temperature and pressure.
B)Ionic compounds do not contain any covalent bonds.
C)Ionic compounds contain the same number of positive ions as negative ions.
D)The chemical formula for an ionic compound must show a nonzero net charge.
A)Essentially all ionic compounds are solids at room temperature and pressure.
B)Ionic compounds do not contain any covalent bonds.
C)Ionic compounds contain the same number of positive ions as negative ions.
D)The chemical formula for an ionic compound must show a nonzero net charge.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
74
The chemical formula for potassium peroxide is
A)KOH.
B)KO2.
C)K2O.
D)K2O2.
A)KOH.
B)KO2.
C)K2O.
D)K2O2.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
75
What group of elements does the shaded area in the following periodic table indicate? 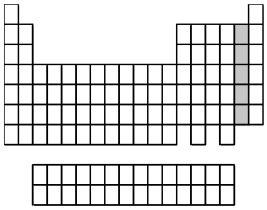
A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
76
The gas Freon-11,CCl3F,contains
A)C4+,Cl-,and F- ions.
B)C4+,Cl3-,and F- ions.
C)C4+ and Cl3F4- ions.
D)CCl3F molecules.
A)C4+,Cl-,and F- ions.
B)C4+,Cl3-,and F- ions.
C)C4+ and Cl3F4- ions.
D)CCl3F molecules.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
77
KH2PO4 is
A)hydropotassium phosphate.
B)potassium dihydrogen phosphate.
C)potassium diphosphate.
D)potassium hydrogen(II)phosphate.
A)hydropotassium phosphate.
B)potassium dihydrogen phosphate.
C)potassium diphosphate.
D)potassium hydrogen(II)phosphate.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
78
What group of elements does the shaded area in the following periodic table indicate? 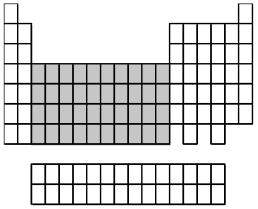
A)alkali metals
B)alkaline earth metals
C)inner transition metals
D)transition metals

A)alkali metals
B)alkaline earth metals
C)inner transition metals
D)transition metals

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
79
The ion NO2- is named
A)nitrate ion.
B)nitrite ion.
C)nitrogen dioxide ion.
D)nitrogen(II)oxide ion.
A)nitrate ion.
B)nitrite ion.
C)nitrogen dioxide ion.
D)nitrogen(II)oxide ion.

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck
80
What group of elements does the shaded area in the following periodic table indicate? 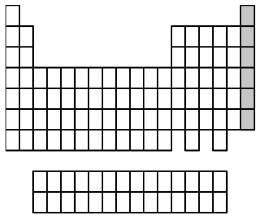
A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

A)alkali metals
B)alkaline earth metals
C)halogens
D)noble gases

Unlock Deck
Unlock for access to all 275 flashcards in this deck.
Unlock Deck
k this deck


