Deck 3: Ionic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 3: Ionic Compounds
1
The term which best describes the crystalline substance that results when a large number of metal atoms transfers electrons to a large number of non-metal atoms is
A)covalent compound.
B)molecule.
C)ionic solid.
D)cation.
E)anion.
A)covalent compound.
B)molecule.
C)ionic solid.
D)cation.
E)anion.
ionic solid.
2
The property that describes the ease with which an atom gives up an electron to form a positive ion is
A)atomic number.
B)electron affinity.
C)electronegativity.
D)ionization energy.
E)none of the above
A)atomic number.
B)electron affinity.
C)electronegativity.
D)ionization energy.
E)none of the above
ionization energy.
3
Which of the following electron configurations is most stable?
A)1s2 2s2
B)1s2 2s2 2p2
C)1s2 2s2 2p3
D)1s2 2s2 2p4
E)1s2 2s2 2p6
A)1s2 2s2
B)1s2 2s2 2p2
C)1s2 2s2 2p3
D)1s2 2s2 2p4
E)1s2 2s2 2p6
1s2 2s2 2p6
4
When an atom donates an electron,that electron
A)is lost for all time.
B)is acquired by another atom which becomes an anion.
C)is acquired by another atom which becomes a cation.
D)neutralizes a proton to form a neutron.
E)pairs with another electron to form a covalent bond.
A)is lost for all time.
B)is acquired by another atom which becomes an anion.
C)is acquired by another atom which becomes a cation.
D)neutralizes a proton to form a neutron.
E)pairs with another electron to form a covalent bond.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following pairs will form ionic bonds with one another?
A)N,C
B)Na,Ca
C)Cs,Br
D)S,Cl
A)N,C
B)Na,Ca
C)Cs,Br
D)S,Cl

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
The statement that best describes the formation of an ionic compound is:
A)Electrons are transferred from a metal to a non-metal,and the resulting charged particles form a crystalline network.
B)Electrons are transferred from a non-metal to a metal,and the resulting charged particles form a crystalline network.
C)Electrons are shared between two atoms and discrete molecules are formed.
D)Electrons move freely among a network of nuclei in fixed positions.
E)Each atom achieves an octet using electrons provided from an external electrical supply.
A)Electrons are transferred from a metal to a non-metal,and the resulting charged particles form a crystalline network.
B)Electrons are transferred from a non-metal to a metal,and the resulting charged particles form a crystalline network.
C)Electrons are shared between two atoms and discrete molecules are formed.
D)Electrons move freely among a network of nuclei in fixed positions.
E)Each atom achieves an octet using electrons provided from an external electrical supply.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
An element belonging to the alkaline earth family would be expected to have a ________ ionization energy and a ________ electron affinity.
A)large; large
B)large; small
C)small; small
D)small; large
E)none of the above
A)large; large
B)large; small
C)small; small
D)small; large
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
What is the most likely charge on an ion formed by an element with a valence electron configuration of ns2np4?
A)2-
B)1-
C)2+
D)4+
E)6+
A)2-
B)1-
C)2+
D)4+
E)6+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
An element belonging to the halogen family would be expected to have a ________ ionization energy and a ________ electron affinity.
A)large; large
B)large; small
C)small; small
D)small; large
E)none of the above
A)large; large
B)large; small
C)small; small
D)small; large
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the compounds below is most likely to be ionic?
A)SrBr2
B)NO2
C)CBr4
D)H2O
A)SrBr2
B)NO2
C)CBr4
D)H2O

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
Main group elements that are metals usually ________ one or more electrons to form ________,which have a ________ charge.
A)lose; anions; negative
B)lose; cations; negative
C)lose; cations; positive
D)gain; cations; positive
E)gain; anions; negative
A)lose; anions; negative
B)lose; cations; negative
C)lose; cations; positive
D)gain; cations; positive
E)gain; anions; negative

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
A small negatively charged particle formed when an atom gains one or more electrons is called a(an)
A)anion.
B)cation.
C)isotope.
D)nucleus.
E)proton.
A)anion.
B)cation.
C)isotope.
D)nucleus.
E)proton.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
The property defined as the energy released on adding an electron to an isolated gas phase atom is
A)atomic number.
B)electron affinity.
C)electronegativity.
D)ionization energy.
E)none of the above
A)atomic number.
B)electron affinity.
C)electronegativity.
D)ionization energy.
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
One characteristic of a cation is that
A)it has more protons than electrons.
B)it has equal numbers of protons and electrons.
C)it has more electrons than protons.
D)the number of neutrons is related to the number of electrons.
E)the relationship between protons and electrons varies with the cation in question.
A)it has more protons than electrons.
B)it has equal numbers of protons and electrons.
C)it has more electrons than protons.
D)the number of neutrons is related to the number of electrons.
E)the relationship between protons and electrons varies with the cation in question.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
A positively charged particle formed by loss of one or more electrons from an atom is called a(an)
A)anion.
B)cation.
C)isotope.
D)nucleus.
E)proton.
A)anion.
B)cation.
C)isotope.
D)nucleus.
E)proton.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
What is the most likely charge on an ion formed by an element with a valence electron configuration of ns1?
A)7-
B)1-
C)0
D)1+
E)7+
A)7-
B)1-
C)0
D)1+
E)7+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following has the largest ionization energy?
A)Ne
B)Br
C)P
D)Al
E)Ca
A)Ne
B)Br
C)P
D)Al
E)Ca

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
All of the following are properties typical of ionic compounds except
A)exist as crystalline solids at room temperature.
B)conduct electrical current if dissolved in water.
C)form distinct molecules by interaction of specific particles.
D)have very high melting points and boiling points.
E)shatter when crystals are struck.
A)exist as crystalline solids at room temperature.
B)conduct electrical current if dissolved in water.
C)form distinct molecules by interaction of specific particles.
D)have very high melting points and boiling points.
E)shatter when crystals are struck.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
The property defined as the energy required to remove one electron from an atom in the gaseous state is
A)atomic number.
B)electron affinity.
C)ionization energy.
D)electronegativity.
E)none of the above
A)atomic number.
B)electron affinity.
C)ionization energy.
D)electronegativity.
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
Main group elements that are non-metals usually ________ one or more electrons to form ________,which have a ________ charge.
A)lose; anions; negative
B)lose; cations; negative
C)lose; cations; positive
D)gain; cations; positive
E)gain; anions; negative
A)lose; anions; negative
B)lose; cations; negative
C)lose; cations; positive
D)gain; cations; positive
E)gain; anions; negative

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following elements is most likely to form an ion with a -1 charge?
A)Mg
B)Si
C)S
D)Cl
E)Sc
A)Mg
B)Si
C)S
D)Cl
E)Sc

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
Briefly explain how the octet rule determines the charge,both sign and magnitude,of an ion.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following ions does not have the same electron configuration as the noble gas neon?
A)O-2
B)F-
C)Al+3
D)S-2
E)Mg+2
A)O-2
B)F-
C)Al+3
D)S-2
E)Mg+2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
The name of Cu2+ is ________ ion or ________ ion.
A)copper; cupric
B)copper(i); cupric
C)copper(II); cupric
D)copper(I); cuprous
E)copper(II); cuprous
A)copper; cupric
B)copper(i); cupric
C)copper(II); cupric
D)copper(I); cuprous
E)copper(II); cuprous

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
What fourth period element is represented by the dot structure shown? 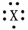
A)K
B)Ca
C)Mn
D)Br
E)Co
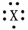
A)K
B)Ca
C)Mn
D)Br
E)Co

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the charge that would be found on a carbide ion and on a nitride ion.Explain why these ions are seldom found in compounds.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
Which pair of elements is most likely to form an ionic compound if allowed to react together?
A)Al and Si
B)Fe and Ca
C)C and F
D)K and Br
E)H and N
A)Al and Si
B)Fe and Ca
C)C and F
D)K and Br
E)H and N

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
How are noble gases related to the octet rule?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
An atom with 3 valence electrons will most likely
A)lose three electrons.
B)gain three electrons.
C)gain five electrons.
D)gain one electron.
E)lose one electron.
A)lose three electrons.
B)gain three electrons.
C)gain five electrons.
D)gain one electron.
E)lose one electron.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
The name of Cl- is
A)chlorine ion.
B)chloride ion.
C)chlorate ion.
D)chlorite ion.
E)diatomic chlorine.
A)chlorine ion.
B)chloride ion.
C)chlorate ion.
D)chlorite ion.
E)diatomic chlorine.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
The name of S2- is
A)sulfur.
B)sulfate ion.
C)sulfite ion.
D)sulfide ion.
E)sulfurous ion.
A)sulfur.
B)sulfate ion.
C)sulfite ion.
D)sulfide ion.
E)sulfurous ion.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
What fourth period element is represented by the dot structure shown?
X:
A)K
B)Ca
C)V
D)As
E)Ti
X:
A)K
B)Ca
C)V
D)As
E)Ti

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
In order to form an octet,an atom of selenium will
A)lose 6 electrons.
B)gain 6 electrons.
C)lose 2 electrons.
D)gain 2 electrons.
A)lose 6 electrons.
B)gain 6 electrons.
C)lose 2 electrons.
D)gain 2 electrons.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following elements is most likely to form an ion with a -2 charge?
A)Mg
B)Si
C)S
D)K
E)Ti
A)Mg
B)Si
C)S
D)K
E)Ti

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
What is the most likely charge on an ion formed by an element with a valence electron configuration of ns2np5?
A)5-
B)1-
C)1+
D)2+
E)5+
A)5-
B)1-
C)1+
D)2+
E)5+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
What is the valence shell electron configuration of the ion formed from an atom of the halogen family?
A)ns2
B)ns2 np2
C)ns2 np4
D)ns2 np6
E)ns2 np8
A)ns2
B)ns2 np2
C)ns2 np4
D)ns2 np6
E)ns2 np8

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
What is the most likely charge on an ion formed by an element with a valence electron configuration of ns2np1?
A)3-
B)1-
C)1+
D)3+
E)5+
A)3-
B)1-
C)1+
D)3+
E)5+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
Which element will form an ion with the greatest positive charge?
A)Al
B)Na
C)Mg
D)Sr
E)P
A)Al
B)Na
C)Mg
D)Sr
E)P

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
The charge on a sulfide ion is ________.
A)3+
B)2+
C)0
D)2-
E)3-
A)3+
B)2+
C)0
D)2-
E)3-

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following elements is most likely to form an ion with a +2 charge?
A)Mg
B)Si
C)S
D)K
E)Br
A)Mg
B)Si
C)S
D)K
E)Br

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
What is the formula for the ionic compound formed between calcium and sulfur?
A)CaS
B)CaSi
C)Ca2S
D)CaS2
E)CaSi2
A)CaS
B)CaSi
C)Ca2S
D)CaS2
E)CaSi2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
What is the formula of a compound formed by the ions M2+ and X3-?
A)M2X3
B)M3X2
C)MX3
D)M2X
E)none of the above
A)M2X3
B)M3X2
C)MX3
D)M2X
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
The formula Ca(NO3)2 tells us that one formula unit of this compound is composed of ________ calcium atoms,________ nitrogen atoms,and ________ oxygen atoms.
A)one; two; six
B)two; two; six
C)one; two; five
D)one; one; five
E)one; one; six
A)one; two; six
B)two; two; six
C)one; two; five
D)one; one; five
E)one; one; six

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
If an element G could react with sulfur to form an ionic compound with formula GS2,the charge on the ion formed by G would be ________.
A)4-
B)2-
C)1+
D)2+
E)4+
A)4-
B)2-
C)1+
D)2+
E)4+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
The correct Roman numeral for the chromium ion in the compound CrCl3 is
A)I
B)II
C)III
D)IV
A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
What is the formula for the ionic compound formed between lithium and bromide?
A)LiB
B)LiBr
C)Li2Br
D)LiBr2
E)Li+Br-
A)LiB
B)LiBr
C)Li2Br
D)LiBr2
E)Li+Br-

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
Which is the correct formula for the ionic compound containing iron(III)ions and oxide ions?
A)FeO
B)FeO2
C)Fe2O2
D)Fe2O3
E)Fe3O2
A)FeO
B)FeO2
C)Fe2O2
D)Fe2O3
E)Fe3O2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
What is the formula of a compound formed by the ions M+ and X3-?
A)MX3
B)M3X
C)M3X3
D)M2X6
E)none of the above
A)MX3
B)M3X
C)M3X3
D)M2X6
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
What is the formula of the ammonium ion?
A)Am-
B)Am+
C)NH4 +
D)NH4+
E)N4H+
A)Am-
B)Am+
C)NH4 +
D)NH4+
E)N4H+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
What is the formula of the sulfite ion?
A)SO3 2-
B)SO4 2-
C)HSO4 2-
D)S 2-
E)none of the above
A)SO3 2-
B)SO4 2-
C)HSO4 2-
D)S 2-
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
The name of Sn2+ is ________ ion or ________ ion.
A)tin; stannous
B)tin(IV); stannic
C)tin(II); stannic
D)tin(IV); stannous
E)tin(II); stannous
A)tin; stannous
B)tin(IV); stannic
C)tin(II); stannic
D)tin(IV); stannous
E)tin(II); stannous

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
The permanganate ion is composed of
A)one atom of magnesium,four atoms of oxygen,and one extra electron.
B)one atom of manganese,four atoms of oxygen,and one extra electron.
C)four atoms of magnesium,four atoms of oxygen,and one extra electron.
D)four atoms of manganese,four atoms of oxygen,and one extra electron.
E)one manganese(II)ion,four oxide ions,and two extra electrons.
A)one atom of magnesium,four atoms of oxygen,and one extra electron.
B)one atom of manganese,four atoms of oxygen,and one extra electron.
C)four atoms of magnesium,four atoms of oxygen,and one extra electron.
D)four atoms of manganese,four atoms of oxygen,and one extra electron.
E)one manganese(II)ion,four oxide ions,and two extra electrons.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
The formula PO4 3- means that this ion is composed of
A)one atom of phosphorus,one atom of oxygen,and three extra electrons.
B)four atoms of phosphorus,four atoms or oxygen,and three extra electrons.
C)one atom of phosphorus,four atoms of oxygen,and three electrons have been lost.
D)one atom of phosphorus,four atoms of oxygen,and three extra electrons.
E)four atoms of phosphorus,four atoms of oxygen,and three electrons have been lost.
A)one atom of phosphorus,one atom of oxygen,and three extra electrons.
B)four atoms of phosphorus,four atoms or oxygen,and three extra electrons.
C)one atom of phosphorus,four atoms of oxygen,and three electrons have been lost.
D)one atom of phosphorus,four atoms of oxygen,and three extra electrons.
E)four atoms of phosphorus,four atoms of oxygen,and three electrons have been lost.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
What is the formula of the carbonate ion?
A)CO2 3-
B)CO3 2-
C)C2O4 2-
D)C2O4 -1
E)C2H3O2 -1
A)CO2 3-
B)CO3 2-
C)C2O4 2-
D)C2O4 -1
E)C2H3O2 -1

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following formulas in incorrect for a cobalt(III)compound?
A)CoCO3
B)CoCl3
C)Co2O3
D)CoPO4
A)CoCO3
B)CoCl3
C)Co2O3
D)CoPO4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
A formula unit of the ionic compound copper(II)carbonate consists of ________ copper(II)ions and ________ carbonate ions.
A)one; one
B)one; two
C)two; one
D)two; two
E)some other combination of ions
A)one; one
B)one; two
C)two; one
D)two; two
E)some other combination of ions

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
What is the formula of the nitrate ion?
A)NO3 2-
B)NO 3-
C)NO2 -1
D)NO3 -1
E)none of the above
A)NO3 2-
B)NO 3-
C)NO2 -1
D)NO3 -1
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
The HCO3 -1 ion is called ________.
A)hydrogen carbonate
B)hydrogen carbide
C)carbide
D)carbonate
E)carbonite
A)hydrogen carbonate
B)hydrogen carbide
C)carbide
D)carbonate
E)carbonite

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
A formula unit of ammonium sulfate consists of ________ ammonium ions and ________ sulfate ions.
A)one; two
B)two; one
C)two; three
D)three; two
E)four; four
A)one; two
B)two; one
C)two; three
D)three; two
E)four; four

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
The main group element E reacts with chlorine to form an ionic compound with the formula ECl2.The element E is a member of what group in the Periodic Table?
A)1A
B)2A
C)6A
D)7A
E)none of the above
A)1A
B)2A
C)6A
D)7A
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following formulas represents a compound that is a base?
A)CaSO4
B)NH4Cl
C)Mg(OH)2
D)H2
E)none of the above
A)CaSO4
B)NH4Cl
C)Mg(OH)2
D)H2
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
The name of the compound with formula NaMnO4 is
A)sodium magnesium oxide.
B)sodium magnesium tetraoxide.
C)sodium manganate.
D)sodium permanganate.
E)sodium manganese tetraoxide.
A)sodium magnesium oxide.
B)sodium magnesium tetraoxide.
C)sodium manganate.
D)sodium permanganate.
E)sodium manganese tetraoxide.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
One definition of a base is a substance that provides which ion in water solution?
A)Na+
B)H+
C)OH-
D)H3O+
E)NH4+
A)Na+
B)H+
C)OH-
D)H3O+
E)NH4+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following formulas represents a compound that is an acid?
A)CaSO4
B)NH4Cl
C)Mg(OH)2
D)H2O
E)H3PO4
A)CaSO4
B)NH4Cl
C)Mg(OH)2
D)H2O
E)H3PO4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
What is the name of AlCl3?
A)aluminum chloride
B)aluminum(III)chloride
C)aluminum carbide
D)aluminum trichloride
E)aluminum tricarbide
A)aluminum chloride
B)aluminum(III)chloride
C)aluminum carbide
D)aluminum trichloride
E)aluminum tricarbide

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
The formula for the compound chromium(II)nitrate is ________.
A)C2NO3
B)Cr2NO3
C)CrNO3
D)Cr(NO3)2
E)CrNO2
A)C2NO3
B)Cr2NO3
C)CrNO3
D)Cr(NO3)2
E)CrNO2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
Iron pyrite (fool's gold)is iron(II)sulfide.What is its formula?
A)FeS
B)FeSO3
C)FeSO4
D)Fe2S3
E)Fe2(SO3)3
A)FeS
B)FeSO3
C)FeSO4
D)Fe2S3
E)Fe2(SO3)3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
What is the formula for calcium phosphate?
A)Ca3(PO4)2
B)Ca2PO4
C)Ca3PO4
D)Ca2(PO3)3
E)Ca4(PO3)3
A)Ca3(PO4)2
B)Ca2PO4
C)Ca3PO4
D)Ca2(PO3)3
E)Ca4(PO3)3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
The formula for potassium dichromate is ________.
A)KCr2O7
B)K2Cr2O7
C)K2CrO4
D)PCr2 O7
E)PCrO4
A)KCr2O7
B)K2Cr2O7
C)K2CrO4
D)PCr2 O7
E)PCrO4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
One definition of an acid is a substance that provides which ion in water solution?
A)Na+
B)H+
C)OH-
D)NH4+
E)none of the above
A)Na+
B)H+
C)OH-
D)NH4+
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
What is the name of K2S?
A)dipotassium sulfide
B)potassium disulfide
C)potassium(II)sulfide
D)potassium sulfide
E)none of the above
A)dipotassium sulfide
B)potassium disulfide
C)potassium(II)sulfide
D)potassium sulfide
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
What is the name of Mg3(PO4)2?
A)trimagnesium diphosphate
B)magnesium diphosphate
C)trimagnesium phosphate
D)magnesium phosphate
E)magnesium phosphorus oxide
A)trimagnesium diphosphate
B)magnesium diphosphate
C)trimagnesium phosphate
D)magnesium phosphate
E)magnesium phosphorus oxide

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
What is the name of SnCl2?
A)ditin chloride
B)tin dichloride
C)tin(II)chloride
D)tin chloride
E)strontium chloride
A)ditin chloride
B)tin dichloride
C)tin(II)chloride
D)tin chloride
E)strontium chloride

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
The formula (NH4)3PO4 indicates that one formula unit of this compound is composed of
A)three atoms of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,four atoms of oxygen,and six extra electrons.
B)three atoms of nitrogen,twelve atoms of hydrogen,four atoms of phosphorus,and four atoms of oxygen.
C)one atom of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
D)three atoms of nitrogen,seven atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
E)three atoms of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
A)three atoms of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,four atoms of oxygen,and six extra electrons.
B)three atoms of nitrogen,twelve atoms of hydrogen,four atoms of phosphorus,and four atoms of oxygen.
C)one atom of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
D)three atoms of nitrogen,seven atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
E)three atoms of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
The formula for ammonium hydroxide is ________.
A)OHNH4
B)NH4NO3
C)NH4O
D)NH4OH
E)Al(OH)3
A)OHNH4
B)NH4NO3
C)NH4O
D)NH4OH
E)Al(OH)3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


