Deck 4: Molecular Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 4: Molecular Compounds
1
A chemical bond formed when two atoms share two pairs of electrons is a ________ bond; it is best described as ________.
A)double; covalent
B)double; ionic
C)single; covalent
D)single; ionic
E)triple; covalent
A)double; covalent
B)double; ionic
C)single; covalent
D)single; ionic
E)triple; covalent
double; covalent
2
In a covalent compound the bond length can be defined as
A)the distance between any two pairs of electrons.
B)the distance between the two largest atoms.
C)the distance between two nuclei when the repulsion is greatest.
D)the distance between two nuclei when the attraction is greatest.
E)the distance between two nuclei when repulsion and attraction are balanced.
A)the distance between any two pairs of electrons.
B)the distance between the two largest atoms.
C)the distance between two nuclei when the repulsion is greatest.
D)the distance between two nuclei when the attraction is greatest.
E)the distance between two nuclei when repulsion and attraction are balanced.
the distance between two nuclei when repulsion and attraction are balanced.
3
Which representation of a hydrogen molecule is not correct?
A)H=H
B)H2
C)H:H
D)HH
E)none of the above
A)H=H
B)H2
C)H:H
D)HH
E)none of the above
H=H
4
The element in the list given that is most likely to form a coordinate covalent bond is
A)H.
B)C.
C)K.
D)Fe.
E)Ca.
A)H.
B)C.
C)K.
D)Fe.
E)Ca.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Which group in the Periodic Table is most likely to contain the element X in the molecule whose dot structure is shown? 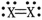
A)2A
B)3A
C)4A
D)5A
E)6A
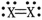
A)2A
B)3A
C)4A
D)5A
E)6A

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
Which element is most likely to be "X" in the diatomic molecule shown? 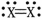
A)nitrogen
B)oxygen
C)fluorine
D)hydrogen
E)helium
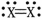
A)nitrogen
B)oxygen
C)fluorine
D)hydrogen
E)helium

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
When two atoms share one or more pairs of electrons,a covalent bond is formed.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
A chemical bond formed when two atoms share three pairs of electrons is a ________ bond; it is best described as ________.
A)double; covalent
B)single; covalent
C)triple; covalent
D)double; ionic
E)triple; ionic
A)double; covalent
B)single; covalent
C)triple; covalent
D)double; ionic
E)triple; ionic

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
For the structure shown,the most likely elements are X = ________ and Y = ________. 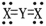
A)carbon; oxygen
B)carbon; hydrogen
C)nitrogen; oxygen
D)oxygen; carbon
E)oxygen; hydrogen
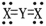
A)carbon; oxygen
B)carbon; hydrogen
C)nitrogen; oxygen
D)oxygen; carbon
E)oxygen; hydrogen

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
Which group contains only elements which normally exist as diatomic molecules?
A)nitrogen; sulfur,bromine
B)helium; neon,argon
C)nitrogen; oxygen,fluorine
D)hydrogen; lithium,sodium
E)oxygen; phosphorus,germanium
A)nitrogen; sulfur,bromine
B)helium; neon,argon
C)nitrogen; oxygen,fluorine
D)hydrogen; lithium,sodium
E)oxygen; phosphorus,germanium

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
A chemical bond formed when two atoms share one pair of electrons is a ________ bond; it is best described as ________.
A)double; covalent
B)double; ionic
C)single; covalent
D)single; ionic
E)triple; covalent
A)double; covalent
B)double; ionic
C)single; covalent
D)single; ionic
E)triple; covalent

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
A triple bond involves the sharing of ________ pairs of electrons between the atoms
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
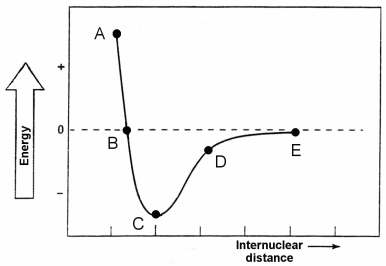
Which point identifies the bond length between the two atoms of the diatomic molecule whose potential energy is shown on the graph?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14

Which point identifies the maximum repulsion between the two atoms of the diatomic molecule whose potential energy is shown in the graph?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
When a non-metal atom bonds with another non-metal atom,an ionic bond is formed.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
Which element is most likely to form three covalent bonds?
A)C
B)Si
C)P
D)S
E)Se
A)C
B)Si
C)P
D)S
E)Se

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
A chemical bond formed between two identical atoms is a(an)________ bond.
A)atomic
B)covalent
C)hydrogen
D)ionic
E)molecular
A)atomic
B)covalent
C)hydrogen
D)ionic
E)molecular

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
For the dot structure shown the most likely elements are X =________ and Y = ________. 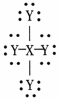
A)carbon; hydrogen
B)carbon; fluorine
C)carbon; oxygen
D)hydrogen; carbon
E)fluorine; carbon
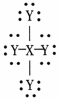
A)carbon; hydrogen
B)carbon; fluorine
C)carbon; oxygen
D)hydrogen; carbon
E)fluorine; carbon

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
A chemical bond formed when two atoms share six electrons is a ________ bond; it is best described as ________.
A)double; covalent
B)double; ionic
C)single; covalent
D)single; ionic
E)triple; covalent
A)double; covalent
B)double; ionic
C)single; covalent
D)single; ionic
E)triple; covalent

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
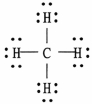
Which representation of a methane molecule is not correct? (A methane molecule is composed of one carbon atom and four hydrogen atoms. )
A)CH4
B)
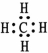
C)
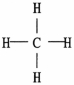
D)
E)none of the above
A)CH4
B)
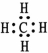
C)

D)

E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
According to VSEPR theory,a molecule with three charge clouds including one lone pair would have a ________ shape.
A)bent
B)linear
C)tetrahedral
D)trigonal planar t
E)pyramidal
A)bent
B)linear
C)tetrahedral
D)trigonal planar t
E)pyramidal

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
The element least likely to obey the octet rule in forming chemical bonds is
A)boron.
B)carbon.
C)nitrogen.
D)oxygen.
E)fluorine.
A)boron.
B)carbon.
C)nitrogen.
D)oxygen.
E)fluorine.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
What is the molecular geometry of PH3?
A)linear
B)trigonal planar
C)tetrahedral
D)bent
E)trigonal pyramidal
A)linear
B)trigonal planar
C)tetrahedral
D)bent
E)trigonal pyramidal

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
The covalent bonding model is most useful in describing which of the following compounds?
A)CoCl2
B)MgCl2
C)NiCl2
D)SCl2
E)none of the above
A)CoCl2
B)MgCl2
C)NiCl2
D)SCl2
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
Which set of properties would identify an unknown white solid as a molecular compound?
I.Contains a metal.
II.Has a definite crystal structure.
III.Dissolves in water,but not in organic liquids.
IV.Melts at 80 degrees Celsius.
V.Does not conduct electricity when melted.
A)I,II,III
B)I,III,V
C)II,III,IV
D)II,IV,V
E)I,IV,V
I.Contains a metal.
II.Has a definite crystal structure.
III.Dissolves in water,but not in organic liquids.
IV.Melts at 80 degrees Celsius.
V.Does not conduct electricity when melted.
A)I,II,III
B)I,III,V
C)II,III,IV
D)II,IV,V
E)I,IV,V

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
The water molecule has a ________ geometry because its central atom has ________ bonds and ________ lone pairs of electrons.
A)bent; two; two
B)linear; two; two
C)pyramidal; three; one
D)tetrahedral; four; zero
E)planar triangular; three; one
A)bent; two; two
B)linear; two; two
C)pyramidal; three; one
D)tetrahedral; four; zero
E)planar triangular; three; one

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
A molecule in which the central atom has no lone pairs and forms four single bonds is said to have a ________ shape.
A)bent
B)linear
C)planar
D)pyramidal
E)tetrahedral
A)bent
B)linear
C)planar
D)pyramidal
E)tetrahedral

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
How many double bonds are there in a molecule of SF2?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
The total number of valence electrons in a molecule of SOF2 is ________.
A)26
B)24
C)18
D)20
E)22
A)26
B)24
C)18
D)20
E)22

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
The number of valence electrons in the acetic acid molecule (CH3CO2H)is ________.
A)0
B)8
C)16
D)24
E)32
A)0
B)8
C)16
D)24
E)32

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
In a Lewis dot structure the electrons which complete an octet but are not located between two atoms are referred to as
A)bonding pairs.
B)delta minus electrons.
C)excess electrons.
D)filled shells.
E)lone pairs.
A)bonding pairs.
B)delta minus electrons.
C)excess electrons.
D)filled shells.
E)lone pairs.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
A molecule in which the central atom forms one double bond and two single bonds is said to have a ________ shape.
A)bent
B)linear
C)trigonal planar
D)pyramidal
E)tetrahedral
A)bent
B)linear
C)trigonal planar
D)pyramidal
E)tetrahedral

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
Which property could describe a covalent compound?
A)It conducts electricity when melted.
B)It is a gas at room temperature.
C)It is composed of a non-metal and a metal.
D)It conducts electricity when dissolved in water.
E)none of the above
A)It conducts electricity when melted.
B)It is a gas at room temperature.
C)It is composed of a non-metal and a metal.
D)It conducts electricity when dissolved in water.
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
The molecule SiCl4 has a ________ shape.
A)bent
B)linear
C)planar
D)pyramidal
E)tetrahedral
A)bent
B)linear
C)planar
D)pyramidal
E)tetrahedral

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
Explain why it is unlikely that an organic molecule would have an odd number of valence electrons.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Covalent compounds form distinct molecules,and therefore may exist as gases,liquids,or solids at room temperature,depending on the characteristics of the compound.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
Which property is most closely associated with covalent molecules?
A)It is a gas at room temperature.
B)It conducts electricity when dissolved in water.
C)It has a very high melting point.
D)It is composed of a metal and a nonmetal.
E)none of these.
A)It is a gas at room temperature.
B)It conducts electricity when dissolved in water.
C)It has a very high melting point.
D)It is composed of a metal and a nonmetal.
E)none of these.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
In forming covalent bonds where the octet rule is obeyed,sulfur usually forms ________ bonds and chlorine usually forms ________ bonds.
A)one; one
B)two; two
C)one; two
D)two; one
E)six; seven
A)one; one
B)two; two
C)one; two
D)two; one
E)six; seven

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
The smallest possible unit of a covalent compound is a(an)
A)atom.
B)cation.
C)formula unit.
D)molecule.
E)polyatomic ion.
A)atom.
B)cation.
C)formula unit.
D)molecule.
E)polyatomic ion.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
A molecule in which the central atom forms three single bonds and has one lone pair is said to have a ________ shape.
A)bent
B)linear
C)planar
D)pyramidal
E)tetrahedral
A)bent
B)linear
C)planar
D)pyramidal
E)tetrahedral

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
A section of the Periodic Table containing main group elements is shown.Which bond would be least polar? 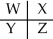
A)WX
B)YZ
C)WZ
D)WY
E)cannot be determined without more specific information
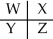
A)WX
B)YZ
C)WZ
D)WY
E)cannot be determined without more specific information

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the molecule SiCl4.The electronegativity values for Si and Cl are 1.8 and 3.0,respectively.Based on these values and on consideration of molecular geometry,the Si-Cl bond is ________ and the molecule is ________.
A)polar; polar
B)nonpolar; nonpolar
C)polar; nonpolar
D)nonpolar; polar
E)none of the above
A)polar; polar
B)nonpolar; nonpolar
C)polar; nonpolar
D)nonpolar; polar
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
Explain how it is possible for CCl4 to have polar bonds but be a non-polar molecule.A diagram may be helpful in your answer,but it must be explained.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
The VSEPR model or molecular structure requires a knowledge of ________ to predict the geometry of an atom in a molecule.
A)the number of atoms bonded to the atom of interest
B)the total number of atoms in the molecule
C)the number of electron pairs on the atom of interest
D)both A and C
E)none of the above
A)the number of atoms bonded to the atom of interest
B)the total number of atoms in the molecule
C)the number of electron pairs on the atom of interest
D)both A and C
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
A section of the Periodic Table containing main group elements is shown.If the elements W,X,Y,and Z have electronegativity values of 1.0,2.0,2.5,and 3.5,respectively,which bond is ionic?
A)WY
B)WZ
C)XY
D)XZ
E)YZ
A)WY
B)WZ
C)XY
D)XZ
E)YZ

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
What is the systematic name of ICl3?
A)iodine chloride
B)iodine(III)chloride
C)triiodine chloride
D)iodine trichloride
E)tri(iodine chloride)
A)iodine chloride
B)iodine(III)chloride
C)triiodine chloride
D)iodine trichloride
E)tri(iodine chloride)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the Lewis structure for CCl4.What is the molecular geometry of this compound? Is the molecule polar or nonpolar?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
A molecule that contains three identical polar bonds to the central atom will be ________.
A)nonpolar if the geometry is planar triangular
B)polar in all cases
C)nonpolar in all cases
D)impossible to tell the polarity
E)either polar or nonpolar depending on the identity of the atoms bonded to the central atom
A)nonpolar if the geometry is planar triangular
B)polar in all cases
C)nonpolar in all cases
D)impossible to tell the polarity
E)either polar or nonpolar depending on the identity of the atoms bonded to the central atom

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
The carbon dioxide molecule is linear.The electronegativities of C and O are 2.5 and 3.5,respectively.Based on these values and on consideration of molecular geometry,the C-O bond is ________ and the molecule is ________.
A)polar; polar
B)nonpolar; nonpolar
C)polar; nonpolar
D)nonpolar; polar
E)none of the above
A)polar; polar
B)nonpolar; nonpolar
C)polar; nonpolar
D)nonpolar; polar
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
A bond where the electrons are shared equally is called a(an)________ bond.
A)polar covalent
B)coordinate covalent
C)nonpolar covalent
D)ionic
E)none of the above
A)polar covalent
B)coordinate covalent
C)nonpolar covalent
D)ionic
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
The formula for sulfur hexabromide is ________.
A)SF6
B)SBr6
C)SBr4
D)SiBr6
E)SiB6
A)SF6
B)SBr6
C)SBr4
D)SiBr6
E)SiB6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following molecules has a CCH bond angle of 180°?
A)HCCH
B)H2CCH2
C)H3CCH3
D)H3CCHO
E)none of them
A)HCCH
B)H2CCH2
C)H3CCH3
D)H3CCHO
E)none of them

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Which element listed is the least electronegative?
A)nitrogen
B)hydrogen
C)oxygen
D)fluorine
E)chlorine
A)nitrogen
B)hydrogen
C)oxygen
D)fluorine
E)chlorine

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
Consider a bent molecule,such as H2Se,in which the central atom has two lone pairs of electrons.The electronegativities of H and Se are 2.1 and 2.4,respectively.Based on these values and on consideration of molecular geometry,the H-Se bond can be considered almost ________ and the molecule is ________.
A)polar; polar
B)nonpolar; nonpolar
C)polar; nonpolar
D)nonpolar; polar
E)none of the above
A)polar; polar
B)nonpolar; nonpolar
C)polar; nonpolar
D)nonpolar; polar
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
A section of the Periodic Table containing main group elements is shown.If the elements W,X,Y,and Z have electronegativity values of 1.0,2.0,2.5,and 3.5,respectively,which bond is the least polar?
A)WX
B)WZ
C)XY
D)XZ
E)YZ
A)WX
B)WZ
C)XY
D)XZ
E)YZ

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
A bond where the electrons are shared unequally is called a(an)________ bond.
A)polar covalent
B)coordinate covalent
C)nonpolar covalent
D)ionic
E)none of the above
A)polar covalent
B)coordinate covalent
C)nonpolar covalent
D)ionic
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
The bond angle in the molecule H2S is ________ because the ________.
A)exactly 109.5°; S atom has four charge clouds
B)greater than 109.5°; lone pairs allow the bond angle to expand
C)less than 109.5°; lone pairs force the hydrogen atoms closer together
D)exactly 120°; lone pairs are counted as one charge cloud
E)exactly 180°; the S atom has two bonds
A)exactly 109.5°; S atom has four charge clouds
B)greater than 109.5°; lone pairs allow the bond angle to expand
C)less than 109.5°; lone pairs force the hydrogen atoms closer together
D)exactly 120°; lone pairs are counted as one charge cloud
E)exactly 180°; the S atom has two bonds

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
Although noble gases do not normally form covalent compounds,XeO3 has been prepared.The systematic name of this compound is
A)trioxy xenon.
B)trixenon trioxide.
C)xenon trioxide.
D)xenon(III)oxide.
E)none of the above
A)trioxy xenon.
B)trixenon trioxide.
C)xenon trioxide.
D)xenon(III)oxide.
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
Which element listed is the most electronegative?
A)aluminum
B)bromine
C)chlorine
D)iodine
E)sodium
A)aluminum
B)bromine
C)chlorine
D)iodine
E)sodium

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
The formula for phosphorus pentafluoride is ________.
A)PhF5
B)PF5
C)P5F
D)(PF)5
E)P5F5
A)PhF5
B)PF5
C)P5F
D)(PF)5
E)P5F5

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
Match the following.
dinitrogen tetroxide
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
dinitrogen tetroxide
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
The compound BrF3 would be called
A)boron trifluoride.
B)triboron fluoride.
C)tri (bromine fluoride).
D)tribromine fluoride.
E)bromine trifluoride.
A)boron trifluoride.
B)triboron fluoride.
C)tri (bromine fluoride).
D)tribromine fluoride.
E)bromine trifluoride.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
If SiCl4 is named as a covalent compound,what would it be called?
A)silicon chloride
B)chlorosilicate
C)silicon tetrachloride
D)sulfur chloride
E)sulfur tetrachloride
A)silicon chloride
B)chlorosilicate
C)silicon tetrachloride
D)sulfur chloride
E)sulfur tetrachloride

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
Match the following.
sulfur dioxide
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
sulfur dioxide
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
Match the following.
carbon monoxide
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
carbon monoxide
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
Match the following.
phosphorus trichloride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
phosphorus trichloride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
Match the following.
carbon tetrachloride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
carbon tetrachloride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
Match the following.
iodine heptafluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
iodine heptafluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
Match the following.
methane
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
methane
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
Match the following.
bromine pentafluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
bromine pentafluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
Match the following.
ammonia
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
ammonia
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
Match the following.
oxygen difluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
oxygen difluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
Match the following.
iodine monochloride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
iodine monochloride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
The formula for carbon disulfide is ________.
A)CS2
B)C2S
C)CSi2
D)CaS2
E)(CS)2
A)CS2
B)C2S
C)CSi2
D)CaS2
E)(CS)2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
Match the following.
sulfur hexafluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3
sulfur hexafluoride
A)OF2
B)BrF5
C)CCI4
D)N2O4
E)SF6
F)CO
G)ICl
H)SO2
I)CH4
J)IF7
K)PCI3
L)NH3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


