Deck 10: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/102
Play
Full screen (f)
Deck 10: Acids and Bases
1
The base forms a new ________ bond in a Brønsted-Lowry acid-base reaction.
A)covalent
B)aquo
C)hydrogen
D)ionic
E)metallic
A)covalent
B)aquo
C)hydrogen
D)ionic
E)metallic
covalent
2
A Brønsted-Lowry base is a substance which
A)produces hydrogen ions in aqueous solution.
B)produces hydroxide ions in aqueous solution.
C)donates protons to other substances.
D)accepts protons from other substances.
E)accepts hydronium ions from other substances.
A)produces hydrogen ions in aqueous solution.
B)produces hydroxide ions in aqueous solution.
C)donates protons to other substances.
D)accepts protons from other substances.
E)accepts hydronium ions from other substances.
accepts protons from other substances.
3
Which of the following compounds is a salt?
A)HBr
B)KNO3
C)H2SO4
D)NaOH
E)C6H12O6
A)HBr
B)KNO3
C)H2SO4
D)NaOH
E)C6H12O6
KNO3
4
What is the conjugate base of HSO4-?
A)SO42-
B)H2SO4
C)H3O+
D)OH-
E)H2SO3
A)SO42-
B)H2SO4
C)H3O+
D)OH-
E)H2SO3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following reaction: NO2- + HCO3- 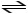
CO3-2 + HNO2
Identify the acid,base,conjugate acid and conjugate base.
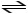
CO3-2 + HNO2
Identify the acid,base,conjugate acid and conjugate base.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
6
When acids and bases react the product other than water is a
A)hydrogen ion.
B)hydroxide ion.
C)hydronium ion.
D)metal.
E)salt.
A)hydrogen ion.
B)hydroxide ion.
C)hydronium ion.
D)metal.
E)salt.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following cannot act as a Brønsted base?
A)HCO3-
B)CO32-
C)NH3
D)NH2-
E)NH4+
A)HCO3-
B)CO32-
C)NH3
D)NH2-
E)NH4+

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a diprotic acid?
A)acetic acid
B)hydrochloric acid
C)phosphoric acid
D)sulfuric acid
E)nitric acid
A)acetic acid
B)hydrochloric acid
C)phosphoric acid
D)sulfuric acid
E)nitric acid

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
9
C5H5N + H2CO3 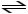
C5H6 N+ + HCO3-
In the reaction shown,the conjugate acid of C5H5N is ________.
A)C5H5N
B)H2CO3
C)C5H6N+
D)HCO3-
E)H3O+
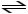
C5H6 N+ + HCO3-
In the reaction shown,the conjugate acid of C5H5N is ________.
A)C5H5N
B)H2CO3
C)C5H6N+
D)HCO3-
E)H3O+

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement concerning Arrhenius acid-base theory is not correct?
A)An Arrhenius acid produces hydrogen ions in water solution.
B)An Arrhenius base produces hydroxide ions in water solution.
C)A neutralization reaction produces water plus a salt.
D)Acid-base reactions must take place in aqueous solution.
E)none of the above
A)An Arrhenius acid produces hydrogen ions in water solution.
B)An Arrhenius base produces hydroxide ions in water solution.
C)A neutralization reaction produces water plus a salt.
D)Acid-base reactions must take place in aqueous solution.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
11
In the following equation,which of the following is acting as the Bronsted acid?
HClO4 + H2O --> ClO4- + H3O+
A)HClO4
B)H2O
C)ClO4-
D)H3O+
HClO4 + H2O --> ClO4- + H3O+
A)HClO4
B)H2O
C)ClO4-
D)H3O+

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
12
According to Brønsted-Lowry theory,acid-base reactions can be described as ________ reactions.
A)electrolytic
B)electron transfer
C)gas phase
D)nuclear transfer
E)proton transfer
A)electrolytic
B)electron transfer
C)gas phase
D)nuclear transfer
E)proton transfer

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
13
Which compound is manufactured in larger quantities in the U.S.than any other industrial chemical?
A)HCl
B)HNO3
C)H3PO4
D)H2SO4
E)NaOH
A)HCl
B)HNO3
C)H3PO4
D)H2SO4
E)NaOH

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
14
Which pair of compounds is used in the manufacture of fertilizers?
A)HCl and NaOH
B)HCl and H3PO4
C)HNO3 and HCl
D)H2SO4 and H3PO4
E)HNO3 and NaOH
A)HCl and NaOH
B)HCl and H3PO4
C)HNO3 and HCl
D)H2SO4 and H3PO4
E)HNO3 and NaOH

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the reaction: H2SO3 + HCO3- 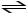
H2CO3 + HSO3-
a)Identify the acid,base,conjugate acid and conjugate base
b)Identify two substances from the reaction which could be used to prepare a buffer.
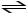
H2CO3 + HSO3-
a)Identify the acid,base,conjugate acid and conjugate base
b)Identify two substances from the reaction which could be used to prepare a buffer.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
16
A Brønsted-Lowry acid is a substance which
A)produces hydrogen ions in aqueous solution.
B)produces hydroxide ions in aqueous solution.
C)donates protons to other substances.
D)accepts protons from other substances.
E)accepts hydronium ions from other substances.
A)produces hydrogen ions in aqueous solution.
B)produces hydroxide ions in aqueous solution.
C)donates protons to other substances.
D)accepts protons from other substances.
E)accepts hydronium ions from other substances.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds is a salt?
A)CH3CO2H
B)NH3
C)NH4NO3
D)Al(OH)3
E)C6H6
A)CH3CO2H
B)NH3
C)NH4NO3
D)Al(OH)3
E)C6H6

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
18
CH3NH2 + HCl 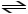
CH3NH3+ + Cl-
A conjugate acid-base pair in the reaction shown is ________ and ________.
A)CH3NH2 and HCl
B)CH3NH2 and Cl-
C)CH3NH3+ and Cl-
D)HCl and Cl-
E)HCl and H3O+
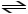
CH3NH3+ + Cl-
A conjugate acid-base pair in the reaction shown is ________ and ________.
A)CH3NH2 and HCl
B)CH3NH2 and Cl-
C)CH3NH3+ and Cl-
D)HCl and Cl-
E)HCl and H3O+

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
19
The H3O+ ion is called the ________ ion.
A)hydroxide
B)hydronium
C)hydrogen
D)protium
E)water
A)hydroxide
B)hydronium
C)hydrogen
D)protium
E)water

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
20
A necessary requirement for a Brønsted base is
A)the presence of water as a reaction medium.
B)the presence of hydroxide in its formula.
C)a lone pair of electrons in its Lewis dot structure.
D)the production of hydronium ion upon reaction with water.
E)the presence of a metal ion in its formula.
A)the presence of water as a reaction medium.
B)the presence of hydroxide in its formula.
C)a lone pair of electrons in its Lewis dot structure.
D)the production of hydronium ion upon reaction with water.
E)the presence of a metal ion in its formula.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
21
Which solution is basic?
A)[H3O+] = 1.0 × 10-4
B)[H3O+] = 1.0 × 10-7
C)[H3O+] = 1.0 × 10-10
D)[OH-] = 1.0 × 10-7
E)[OH-] = 1.0 × 10-10
A)[H3O+] = 1.0 × 10-4
B)[H3O+] = 1.0 × 10-7
C)[H3O+] = 1.0 × 10-10
D)[OH-] = 1.0 × 10-7
E)[OH-] = 1.0 × 10-10

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a weak acid?
A)HNO3
B)H3PO4
C)NH3
D)HCl
E)OH-
A)HNO3
B)H3PO4
C)NH3
D)HCl
E)OH-

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a strong acid?
A)HNO3
B)H3PO4
C)NH4+
D)HCO3-
E)H2O
A)HNO3
B)H3PO4
C)NH4+
D)HCO3-
E)H2O

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
24
What is the conjugate base of water?
A)H2O (l)
B)H3O+ (aq)
C)OH- (aq)
D)H+ (aq)
E)O2- (aq)
A)H2O (l)
B)H3O+ (aq)
C)OH- (aq)
D)H+ (aq)
E)O2- (aq)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a triprotic acid?
A)H3PO4
B)CH3COOH
C)HNO3
D)NH3
E)Al(OH)3
A)H3PO4
B)CH3COOH
C)HNO3
D)NH3
E)Al(OH)3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
26
Which reaction best illustrates the behavior of the weak base H2PO4- in aqueous solution?
A)H2PO4- (aq)+ H2O (l)
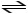
HPO42- (aq)+ H3O+ (aq)
B)H2PO4- (aq)+ H2O (l)
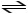
H3PO4 (aq)+ OH- (aq)
C)H2PO4- (aq)
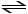
2H+ (aq)+ PO43- (aq)
D)H2PO4- (aq)
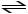
H+ (aq)+ HPO42- (aq)
E)H2PO4- (aq)+ H+ (aq)
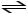
H3PO4 (aq)
A)H2PO4- (aq)+ H2O (l)
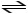
HPO42- (aq)+ H3O+ (aq)
B)H2PO4- (aq)+ H2O (l)
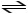
H3PO4 (aq)+ OH- (aq)
C)H2PO4- (aq)
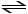
2H+ (aq)+ PO43- (aq)
D)H2PO4- (aq)
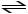
H+ (aq)+ HPO42- (aq)
E)H2PO4- (aq)+ H+ (aq)
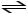
H3PO4 (aq)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
27
Water and HSO4- can either accept protons or donate protons.Such substances are said to be
A)amphoteric.
B)conjugate.
C)diprotic.
D)monoprotic.
E)triprotic.
A)amphoteric.
B)conjugate.
C)diprotic.
D)monoprotic.
E)triprotic.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
28
Which compound has a value of Ka that is close to 10-5?
A)NaCl
B)HNO3
C)CH3CH2CO2H
D)KOH
E)NH3
A)NaCl
B)HNO3
C)CH3CH2CO2H
D)KOH
E)NH3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
29
The classification of an acid or a base as weak or strong is determined by
A)the solubility of the acid or base.
B)the extent of dissociation of the dissolved acid or base.
C)the concentrations of the acid or base.
D)more than one choice is correct.
A)the solubility of the acid or base.
B)the extent of dissociation of the dissolved acid or base.
C)the concentrations of the acid or base.
D)more than one choice is correct.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
30
What is the conjugate acid of water?
A)H2O (l)
B)H3O+ (aq)
C)OH- (aq)
D)H+ (aq)
E)O2- (aq)
A)H2O (l)
B)H3O+ (aq)
C)OH- (aq)
D)H+ (aq)
E)O2- (aq)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following is the strongest weak acid?
A)HCN (Ka = 4.9 × 10-10)
B)HClO (Ka = 3.0 × 10-8)
C)HNO2 (Ka = 4.5 × 10-4)
D)HF (Ka = 6.8 × 10-4)
A)HCN (Ka = 4.9 × 10-10)
B)HClO (Ka = 3.0 × 10-8)
C)HNO2 (Ka = 4.5 × 10-4)
D)HF (Ka = 6.8 × 10-4)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
32
Hydrogen cyanide,HCN,is a weak acid.Which equation best represents its aqueous chemistry?
A)HCN (aq)+ H2O (l)
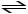
CN- (aq)+ H3O+ (aq)
B)HCN (aq)+ H2O (l)
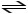
H2CN+ (aq)+ OH- (aq)
C)HCN (aq)
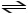
H+ (aq)+ CN- (aq)
D)HCN (aq)
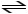
H- (aq)+ CN+ (aq)
E)H2O (l)
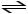
H+ (aq)+ OH- (aq)
A)HCN (aq)+ H2O (l)
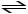
CN- (aq)+ H3O+ (aq)
B)HCN (aq)+ H2O (l)
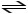
H2CN+ (aq)+ OH- (aq)
C)HCN (aq)
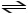
H+ (aq)+ CN- (aq)
D)HCN (aq)
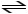
H- (aq)+ CN+ (aq)
E)H2O (l)
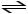
H+ (aq)+ OH- (aq)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
33
Which one of the following is the weakest acid?
A)HCN (Ka = 4.9 × 10-10)
B)HClO (Ka = 3.0 × 10-8)
C)HNO2 (Ka = 4.5 × 10-4)
D)HF (Ka = 6.8 × 10-4)
A)HCN (Ka = 4.9 × 10-10)
B)HClO (Ka = 3.0 × 10-8)
C)HNO2 (Ka = 4.5 × 10-4)
D)HF (Ka = 6.8 × 10-4)

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
34
What is the conjugate acid of HSO4-?
A)SO42-
B)H2SO4
C)H3O+
D)OH-
E)H2SO3
A)SO42-
B)H2SO4
C)H3O+
D)OH-
E)H2SO3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
35
Ammonia reacts with acids because
A)it contains the hydroxide group.
B)it is neutral.
C)it is itself an acid.
D)it is a salt.
E)it contains a lone pair of electrons.
A)it contains the hydroxide group.
B)it is neutral.
C)it is itself an acid.
D)it is a salt.
E)it contains a lone pair of electrons.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
36
At 25°C,the value of Kw is ________.
A)1.00
B)1.00 × 10-7
C)1.00 × 10-14
D)1.00 × 107
E)1.00 × 1014
A)1.00
B)1.00 × 10-7
C)1.00 × 10-14
D)1.00 × 107
E)1.00 × 1014

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
37
Acetic acid is a weak acid in water because it is
A)dilute.
B)only slightly soluble.
C)unable to hold onto its hydrogen ion.
D)only slightly dissociated into ions.
E)completely dissociated into hydronium ions and acetate ions.
A)dilute.
B)only slightly soluble.
C)unable to hold onto its hydrogen ion.
D)only slightly dissociated into ions.
E)completely dissociated into hydronium ions and acetate ions.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement is correct for pure water?
A)Pure water contains equal amounts of hydroxide,[OH-],and hydronium,[H3O+],ions.
B)Pure water contains larger amounts of hydroxide,[OH-],ions than hydronium,[H3O+],ions.
C)Pure water contains larger amounts of hydronium,[H3O+],ions than hydroxide,
[OH-],ions.
D)Pure water is an electrolyte.
E)Pure water contains no ions.
A)Pure water contains equal amounts of hydroxide,[OH-],and hydronium,[H3O+],ions.
B)Pure water contains larger amounts of hydroxide,[OH-],ions than hydronium,[H3O+],ions.
C)Pure water contains larger amounts of hydronium,[H3O+],ions than hydroxide,
[OH-],ions.
D)Pure water is an electrolyte.
E)Pure water contains no ions.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
39
Which compound has a very large value of Ka in aqueous solution?
A)NaCl
B)HNO3
C)H3PO4
D)KOH
E)NH3
A)NaCl
B)HNO3
C)H3PO4
D)KOH
E)NH3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
40
In an aqueous solution that is basic,[H3O+] is ________ than 1.0 × 10-7 and ________ than [OH-].
A)greater; less
B)less; greater
C)greater; greater
D)less; less
E)none of the above
A)greater; less
B)less; greater
C)greater; greater
D)less; less
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements is correct?
A)In a basic solution,[H3O+] < 10-7; [OH-] < 10-7.
B)In a basic solution,[H3O+] > 10-7; [OH-] > 10-7.
C)In a basic solution,[H3O+] > 10-7; [OH-] < 10-7.
D)In a basic solution,[H3O+] < 10-7; [OH-] > 10-7.
E)In a basic solution,[H3O+] > 10-7; [OH-] = 10-7.
A)In a basic solution,[H3O+] < 10-7; [OH-] < 10-7.
B)In a basic solution,[H3O+] > 10-7; [OH-] > 10-7.
C)In a basic solution,[H3O+] > 10-7; [OH-] < 10-7.
D)In a basic solution,[H3O+] < 10-7; [OH-] > 10-7.
E)In a basic solution,[H3O+] > 10-7; [OH-] = 10-7.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
42
What is the pH of a solution in which the hydrogen ion concentration is 5.1 × 10-8 M?
A)6.71
B)1.96 × 10-7
C)8.90
D)5.10
E)7.29
A)6.71
B)1.96 × 10-7
C)8.90
D)5.10
E)7.29

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
43
What is the pH of a solution in which [H3O+] = 3.8 × 10-8 M?
A)7.42
B)6.58
C)3.80
D)1.0 × 10-8
E)2.6 × 10-7
A)7.42
B)6.58
C)3.80
D)1.0 × 10-8
E)2.6 × 10-7

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
44
An increase in solution pH corresponds to
A)an increase in hydronium ion concentration.
B)a decrease in hydronium ion concentration.
C)no change in hydronium ion concentration.
D)a decrease in hydroxide ion concentration.
A)an increase in hydronium ion concentration.
B)a decrease in hydronium ion concentration.
C)no change in hydronium ion concentration.
D)a decrease in hydroxide ion concentration.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
45
What is the value of [H3O+] in a solution with pH = 10.82?
A)6.6 × 10-4 M
B)1.5 × 10-11 M
C)1.03 M
D)10.82 M
E)3.18 M
A)6.6 × 10-4 M
B)1.5 × 10-11 M
C)1.03 M
D)10.82 M
E)3.18 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
46
What is the hydrogen ion concentration in a solution with pH = 2.34?
A)4.57 × 10-3 M
B)2.34 × 10-3 M
C)2.19 × 10-12 M
D)1.17 × 101 M
E)4.27 × 10-12 M
A)4.57 × 10-3 M
B)2.34 × 10-3 M
C)2.19 × 10-12 M
D)1.17 × 101 M
E)4.27 × 10-12 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
47
The pH of a solution with [H3O+] = 5.9 × 10-3 M,to the correct number of significant figures,is ________.
A)5.9 × 10-3
B)2.2
C)2.23
D)1.01
E)2.229
A)5.9 × 10-3
B)2.2
C)2.23
D)1.01
E)2.229

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
48
What is the pH of a solution in which [H3O+] = 1.2 × 10-3 M?
A)1.20
B)2.92
C)11.08
D)12.80
E)8.33 × 10-12
A)1.20
B)2.92
C)11.08
D)12.80
E)8.33 × 10-12

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
49
What is the pH of a solution in which [H3O+] = 4.1 × 10-2 M?
A)12.61
B)1.39
C)1.10
D)12.90
E)4.10
A)12.61
B)1.39
C)1.10
D)12.90
E)4.10

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
50
If the concentration of OH- in an aqueous solution is 1.4 × 10-7 M,the concentration of H3O+ is ________.
A)7.1 × 10+6 M
B)1.0 × 10-7 M
C)1.4 × 10-7 M
D)7.1 × 10-8 M
E)1.3 × 10-8 M
A)7.1 × 10+6 M
B)1.0 × 10-7 M
C)1.4 × 10-7 M
D)7.1 × 10-8 M
E)1.3 × 10-8 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
51
In an aqueous solution that is acidic,[H3O+] is ________ than 1.0 × 10-7 and ________ than [OH-].
A)greater; less
B)less; greater
C)greater; greater
D)less; less
E)none of the above
A)greater; less
B)less; greater
C)greater; greater
D)less; less
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
52
Which example is not acidic?
A)orange juice
B)soil for azaleas with pH of 4.8
C)a solution of NH4NO3 with pH < 7.00
D)lake water that turns blue litmus to red
E)a solution in which [H3O+] = 1.00 × 10-7
A)orange juice
B)soil for azaleas with pH of 4.8
C)a solution of NH4NO3 with pH < 7.00
D)lake water that turns blue litmus to red
E)a solution in which [H3O+] = 1.00 × 10-7

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
53
Which example is not basic?
A)shampoo
B)vinegar
C)window cleaner
D)limewater
E)Mg(OH)2,used in remedies for upset stomach
A)shampoo
B)vinegar
C)window cleaner
D)limewater
E)Mg(OH)2,used in remedies for upset stomach

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
54
The pH of a cup of coffee is measured as 5.45.Express this measurement as [H+],using the correct number of significant figures.
A)3.548 × 10-6 M
B)3.55 × 10-6 M
C)3.5 × 10-6 M
D)4 × 10-6 M
E)2.82 × 105 M
A)3.548 × 10-6 M
B)3.55 × 10-6 M
C)3.5 × 10-6 M
D)4 × 10-6 M
E)2.82 × 105 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
55
If the [H3O+] of a water sample is 1 × 10-4 M,the pH of the sample is ________,and the sample is ________.
A)-4; acidic
B)4; acidic
C)4; basic
D)10; basic
E)-10; basic
A)-4; acidic
B)4; acidic
C)4; basic
D)10; basic
E)-10; basic

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
56
If the concentration of OH- is 1 × 10-2 M,the concentration of H3O+ is ________ M.
A)1 × 100
B)1 × 10-2
C)1 × 10-7
D)1 × 10-12
E)1 × 10-14
A)1 × 100
B)1 × 10-2
C)1 × 10-7
D)1 × 10-12
E)1 × 10-14

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following statements is correct?
A)In an acidic solution,[H3O+] < 10-7; [OH-] < 10-7.
B)In an acidic solution,[H3O+] > 10-7; [OH-] > 10-7.
C)In an acidic solution,[H3O+] > 10-7; [OH-] < 10-7.
D)In an acidic solution,[H3O+] < 10-7; [OH-] > 10-7.
E)In an acidic solution,[H3O+] > 10-7; [OH-] = 10-7.
A)In an acidic solution,[H3O+] < 10-7; [OH-] < 10-7.
B)In an acidic solution,[H3O+] > 10-7; [OH-] > 10-7.
C)In an acidic solution,[H3O+] > 10-7; [OH-] < 10-7.
D)In an acidic solution,[H3O+] < 10-7; [OH-] > 10-7.
E)In an acidic solution,[H3O+] > 10-7; [OH-] = 10-7.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
58
If the [H+] of a water sample is 1 × 10-4 M,the [OH-] is
A)1 × 10-4 M.
B)1 × 10-14 M.
C)1 × 104 M.
D)1 × 10-10 M.
E)none of the above
A)1 × 10-4 M.
B)1 × 10-14 M.
C)1 × 104 M.
D)1 × 10-10 M.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
59
If the concentration of H3O+ in an aqueous solution is 7.6 × 10-9 M,the concentration of OH- is ________.
A)7.6 × 10-23 M
B)1.3 × 10+8 M
C)6.4 × 10-5 M
D)1.3 × 10-6 M
E)7.6 × 10-9 M
A)7.6 × 10-23 M
B)1.3 × 10+8 M
C)6.4 × 10-5 M
D)1.3 × 10-6 M
E)7.6 × 10-9 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
60
If the concentration of H3O+ is 3.5 × 10-3 M,the concentration of OH- is ________ M.
A)2.9 × 10-12
B)1.0 × 10-12
C)1.0 × 10-7
D)3.5 × 10-11
E)10.5 × 10-3
A)2.9 × 10-12
B)1.0 × 10-12
C)1.0 × 10-7
D)3.5 × 10-11
E)10.5 × 10-3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate the hydrogen ion concentration in a solution with pH = 6.35.
A)7.65 M
B)6.35 M
C)4.5 × 10-7 M
D)0.80 M
E)2.2 × 10-8 M
A)7.65 M
B)6.35 M
C)4.5 × 10-7 M
D)0.80 M
E)2.2 × 10-8 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
62
The pH of a 250.mL sample of a buffer solution is 9.85.If 1.0 mL of 6 M HCl is added,the pH of the resulting mixture is closest to ________.
A)0.00
B)1.65
C)7.00
D)9.70
E)10.00
A)0.00
B)1.65
C)7.00
D)9.70
E)10.00

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
63
A buffer solution
A)is a salt solution.
B)maintains pH at 7.00.
C)is a strong base.
D)neutralizes only acids.
E)closely maintains its original pH.
A)is a salt solution.
B)maintains pH at 7.00.
C)is a strong base.
D)neutralizes only acids.
E)closely maintains its original pH.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following pH's corresponds to a strongly basic solution?
A)11.5
B)2.7
C)6.9
D)7.4
E)4.3
A)11.5
B)2.7
C)6.9
D)7.4
E)4.3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
65
The normality of a solution prepared by dissolving 50.0 g of Ca(OH)2 in water to make 250.mL solution is ________ N.
A)1.35
B)2.70
C)3.51
D)5.40
E)7.02
A)1.35
B)2.70
C)3.51
D)5.40
E)7.02

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
66
To prepare a buffer using sodium phosphate,which of the following would also be needed?
A)hydrochloric acid
B)ammonium hydroxide
C)ammonium phosphate
D)phosphoric acid
E)sodium hydroxide
A)hydrochloric acid
B)ammonium hydroxide
C)ammonium phosphate
D)phosphoric acid
E)sodium hydroxide

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
67
The [OH-] and the pH of 0.035 M KOH at 25°C are,respectively,
A)0.035 M and +1.46.
B)0.035 M and -1.46.
C)2.9 × 10-13 M and -12.54.
D)0.035 and +12.54.
E)2.9 × 10-13 M and +12.54.
A)0.035 M and +1.46.
B)0.035 M and -1.46.
C)2.9 × 10-13 M and -12.54.
D)0.035 and +12.54.
E)2.9 × 10-13 M and +12.54.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
68
The normality of a solution prepared by dissolving 25.0 g of Ca(OH)2 in water to make 250.mL solution is ________ N.
A)2.70
B)1.35
C)0.675
D)3.51
E)1.75
A)2.70
B)1.35
C)0.675
D)3.51
E)1.75

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
69
Which of these,if dissolved in 1.0 L of pure water,would produce a buffer solution?
A)0.1 mol NaCl + 0.1 mol KCl
B)0.1 mol H3O+ + 0.1 OH-
C)0.1 mol NaH2PO4 + 0.1 mol Na2HPO4
D)0.1 mol HCl + 0.1 mol NaOH
E)0.1 mol H3O+ + 0.1 mol Cl-
A)0.1 mol NaCl + 0.1 mol KCl
B)0.1 mol H3O+ + 0.1 OH-
C)0.1 mol NaH2PO4 + 0.1 mol Na2HPO4
D)0.1 mol HCl + 0.1 mol NaOH
E)0.1 mol H3O+ + 0.1 mol Cl-

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following solutions is a buffer?
A)a solution of acetic acid and sodium acetate
B)a solution of acetic acid and sodium sulfate
C)a solution of hydrochloric acid and sodium sulfate
D)a solution of hydrochloric acid and sodium acetate
E)a solution of sulfuric acid and sodium sulfate
A)a solution of acetic acid and sodium acetate
B)a solution of acetic acid and sodium sulfate
C)a solution of hydrochloric acid and sodium sulfate
D)a solution of hydrochloric acid and sodium acetate
E)a solution of sulfuric acid and sodium sulfate

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
71
All of the statements regarding equivalents of acids and bases are true except
A)equivalents are the same as moles.
B)equivalents of acid are based on the number of hydrogen ions produced per formula unit of acid.
C)equivalents are used to determine normality of solutions.
D)the equivalent weight of a base is the weight that produces one mole of hydroxide ions.
E)one equivalent of any acid will neutralize one equivalent of any base.
A)equivalents are the same as moles.
B)equivalents of acid are based on the number of hydrogen ions produced per formula unit of acid.
C)equivalents are used to determine normality of solutions.
D)the equivalent weight of a base is the weight that produces one mole of hydroxide ions.
E)one equivalent of any acid will neutralize one equivalent of any base.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
72
Explain the term "amphoteric." Use the hydrogen carbonate ion,HCO3- to illustrate amphoteric behavior.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
73
What is the [H3O+] in a solution with pH = 11.61?
A)1.16 × 10+1 M
B)2.39 × 10+1 M
C)4.07 × 10-3 M
D)1.00 × 10-14 M
E)2.45 × 10-12 M
A)1.16 × 10+1 M
B)2.39 × 10+1 M
C)4.07 × 10-3 M
D)1.00 × 10-14 M
E)2.45 × 10-12 M

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
74
What is the normality of a solution prepared by dissolving 37.5 g citric acid,a triprotic acid with molar mass of 192.14 g,in water to make 250.mL solution?
A)2.34 N
B)0.780 N
C)0.147 N
D)0.0865 N
E)0.288 N
A)2.34 N
B)0.780 N
C)0.147 N
D)0.0865 N
E)0.288 N

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
75
What is the normality of a solution prepared by dissolving 75.0 g citric acid,a triprotic acid with molar mass of 192.14 g,in water to make 250.mL solution?
A)0.173 N
B)0.0576 N
C)0.293 N
D)4.68 N
E)1.56 N
A)0.173 N
B)0.0576 N
C)0.293 N
D)4.68 N
E)1.56 N

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following pH's corresponds to a weakly acidic solution?
A)5.3
B)1.4
C)7.8
D)9.2
E)11.5
A)5.3
B)1.4
C)7.8
D)9.2
E)11.5

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
77
What is the normality of a solution containing 100.g HNO3 in 500.mL of solution?
A)1.26 N
B)1.59 N
C)3.17 N
D)0.500 N
E)0.630 N
A)1.26 N
B)1.59 N
C)3.17 N
D)0.500 N
E)0.630 N

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following pH's corresponds to a neutral solution?
A)7.0
B)1.8
C)6.2
D)8.5
E)14.0
A)7.0
B)1.8
C)6.2
D)8.5
E)14.0

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
79
What is the normality of a solution containing 49 g of H2SO4 in enough water to make 400 mL of solution?
A)2.5 N
B)5.0 N
C)1.0 N
D)10 N
E)0.20 N
A)2.5 N
B)5.0 N
C)1.0 N
D)10 N
E)0.20 N

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
80
All of the following species are involved in the blood buffer system except ________.
A)CO2
B)H2CO3
C)HCO3-
D)CO32-
E)none of the above
A)CO2
B)H2CO3
C)HCO3-
D)CO32-
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck


