Deck 16: Amines
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 16: Amines
1
Which of the following line bond drawings correctly represents N,N-diisopropylamine?
A)
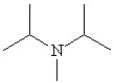
B)
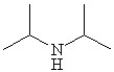
C)
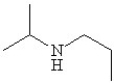
D)
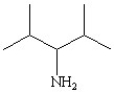
E)
A)

B)

C)

D)

E)


2
Amines are classified by the number of
A)alkyl groups attached to the nitrogen.
B)hydrogens attached to the nitrogen.
C)carbons attached to the carbon bonded to the nitrogen.
D)carbons present in the molecule.
E)none of the above
A)alkyl groups attached to the nitrogen.
B)hydrogens attached to the nitrogen.
C)carbons attached to the carbon bonded to the nitrogen.
D)carbons present in the molecule.
E)none of the above
alkyl groups attached to the nitrogen.
3
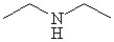
What is the IUPAC name of the compound shown? 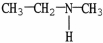
A)isopropylamine
B)propylamine
C)2-propylamine
D)1-methylethylamine
E)N-methylethylamine
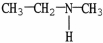
A)isopropylamine
B)propylamine
C)2-propylamine
D)1-methylethylamine
E)N-methylethylamine
N-methylethylamine
4
Amines can be considered organic derivatives of the inorganic compound
A)ammonia.
B)carbon dioxide.
C)sodium hydroxide.
D)water.
E)none of these
A)ammonia.
B)carbon dioxide.
C)sodium hydroxide.
D)water.
E)none of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which molecule is N,N-dimethylpropylamine?
A)C -C
-C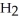 -C
-C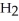 -C
-C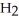 -C
-C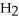 -N
-N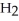
B)
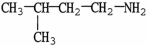
C)
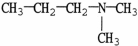
D)
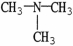
E)
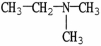
A)C
 -C
-C -C
-C -C
-C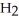 -C
-C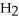 -N
-N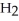
B)
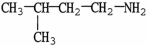
C)
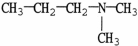
D)
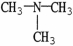
E)
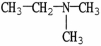

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
Write the correct name for the following and determine if it contains a primary,secondary,or tertiary amine.
A)
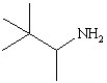
B)
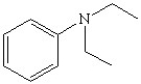
C)
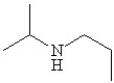
A)

B)

C)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Which molecule shown is trimethylamine?
A)C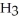 -C
-C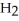 -C
-C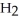 -C
-C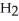 -C
-C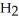 -N
-N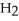
B)
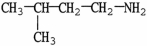
C)
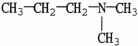
D)
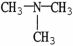
E)
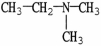
A)C
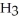 -C
-C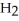 -C
-C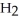 -C
-C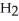 -C
-C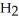 -N
-N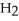
B)
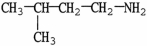
C)
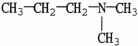
D)
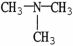
E)
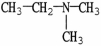

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
All of the following are properties of amines except
A)they frequently have offensive odors.
B)those that can form hydrogen bonds have higher boiling points than expected for their molecular weight.
C)those with low molecular weights are soluble in water.
D)they act as bases in many reactions.
E)All of these are properties of amines.
A)they frequently have offensive odors.
B)those that can form hydrogen bonds have higher boiling points than expected for their molecular weight.
C)those with low molecular weights are soluble in water.
D)they act as bases in many reactions.
E)All of these are properties of amines.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Which molecule shown is N,N-dimethylethylamine?
A) C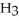 -C
-C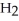 -C
-C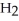 -C
-C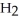 -C
-C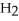 -N
-N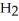
B)
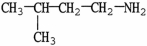
C)
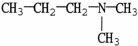
D)
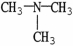
E)
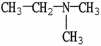
A) C
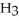 -C
-C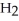 -C
-C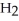 -C
-C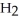 -C
-C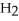 -N
-N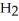
B)
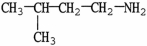
C)
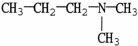
D)
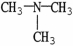
E)
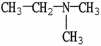

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Amines are most similar in chemical structure and behavior to
A)the hydronium ion.
B)ammonia.
C)sodium hydroxide.
D)a primary alcohol.
E)water.
A)the hydronium ion.
B)ammonia.
C)sodium hydroxide.
D)a primary alcohol.
E)water.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Which molecule is a tertiary amine?
A)
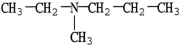
B)
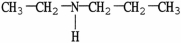
C)
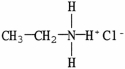
D)
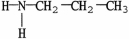
E)
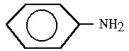
A)
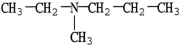
B)
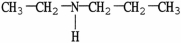
C)

D)
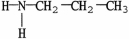
E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct IUPAC name for the following compound? 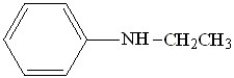
A)ethyl phenyl amine
B)N-ethyl aniline
C)ethyl aniline
D)2-ethyl aniline

A)ethyl phenyl amine
B)N-ethyl aniline
C)ethyl aniline
D)2-ethyl aniline

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
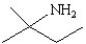
13
Which of the following structures represents the compound 2-amino-2-methylbutane?
A)
B)
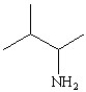
C)
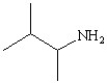
D)
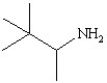
E)
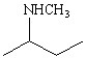
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
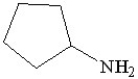
Which of the following molecules is an example of a primary amine?
A)
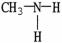
B)
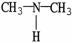
C)
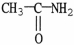
D)
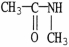
E)
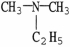
A)
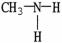
B)
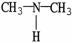
C)
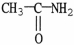
D)
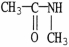
E)
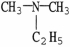

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Which compound is a secondary amine?
A)trimethylamine
B)diethylamine
C)isopropylamine
D)N,N-dimethylethylamine
E)N-ethyl-N-methylpropylamine
A)trimethylamine
B)diethylamine
C)isopropylamine
D)N,N-dimethylethylamine
E)N-ethyl-N-methylpropylamine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Which organic functional group is important for its basic properties?
A)amine
B)aromatic
C)carbonyl
D)hydroxyl
E)phenol
A)amine
B)aromatic
C)carbonyl
D)hydroxyl
E)phenol

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
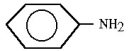
17
What is the correct IUPAC name of the compound shown? 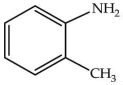
A)N-methyl aniline
B)2-methyl aniline
C)1-methyl aniline
D)1-methyl-2-aniline
E)1-methyl-1-aniline

A)N-methyl aniline
B)2-methyl aniline
C)1-methyl aniline
D)1-methyl-2-aniline
E)1-methyl-1-aniline

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules is an example of a secondary amine?
A)
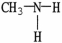
B)
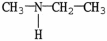
C)
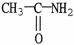
D)
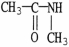
E)
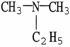
A)
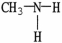
B)
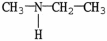
C)
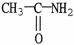
D)
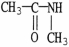
E)
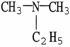

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
When the nitrogen atom in an organic compound has four covalent bonds,it is called a
A)primary amine.
B)secondary amine.
C)tertiary amine.
D)quaternary ammonium ion.
E)tetraammine.
A)primary amine.
B)secondary amine.
C)tertiary amine.
D)quaternary ammonium ion.
E)tetraammine.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Which compound is a primary amine?
A)trimethylamine
B)diethylamine
C)isopropylamine
D)N,N-dimethylethylamine
E)N-ethyl-N-methylpropylamine
A)trimethylamine
B)diethylamine
C)isopropylamine
D)N,N-dimethylethylamine
E)N-ethyl-N-methylpropylamine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
When comparing amine compounds of different classes but similar molar masses,which type will most likely be the highest boiling point?
A)primary amines
B)secondary amines
C)tertiary amines
D)quaternary ammonium salts
A)primary amines
B)secondary amines
C)tertiary amines
D)quaternary ammonium salts

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is a heterocyclic amine?
A)
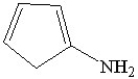
B)
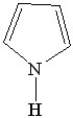
C)
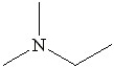
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
Which molecule is a heterocyclic compound?
A)
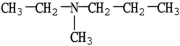
B)
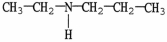
C)
D)
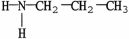
E)none of these
A)
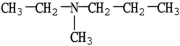
B)
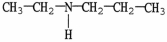
C)

D)
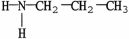
E)none of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
The boiling point of ethylamine is higher than that of propane but lower than that of 1-propanol.This means that
A)a molecule of ethylamine cannot form a hydrogen bond with another molecule of itself.
B)hydrogen bonds between two amino groups are weaker than those between two alcohol groups.
C)the intermolecular forces between two amine molecules are only dipole-dipole.
D)the intermolecular forces between two amine molecules are ionic in nature.
E)the intermolecular forces between two amine molecules are only London dispersion forces.
A)a molecule of ethylamine cannot form a hydrogen bond with another molecule of itself.
B)hydrogen bonds between two amino groups are weaker than those between two alcohol groups.
C)the intermolecular forces between two amine molecules are only dipole-dipole.
D)the intermolecular forces between two amine molecules are ionic in nature.
E)the intermolecular forces between two amine molecules are only London dispersion forces.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Which amine has the highest boiling point?
A)
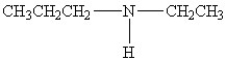
B)
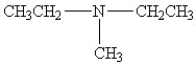
C)
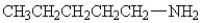
D)
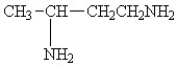
A)

B)

C)
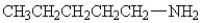
D)
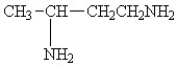

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
When an amine behaves as a base it ________ a hydrogen ion to form a(an)________ ion.
A)loses;hydronium
B)loses;ammonium
C)loses;hydroxide
D)gains;ammonium
E)gains;hydronium
A)loses;hydronium
B)loses;ammonium
C)loses;hydroxide
D)gains;ammonium
E)gains;hydronium

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following compounds contains both a heterocylic amine and an amino group in the same molecule?
A)nicotine
B)caffeine
C)tryptophan
D)quinine
E)purine
A)nicotine
B)caffeine
C)tryptophan
D)quinine
E)purine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
All of the following compounds are amines except
A)amphetamine.
B)histamine.
C)aniline.
D)aspirin.
E)caffeine.
A)amphetamine.
B)histamine.
C)aniline.
D)aspirin.
E)caffeine.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Using dashed lines illustrate how ethyl amine would hydrogen bond with water,increasing its solubility.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Using dashed lines show how two molecules of ethylamine could possibly hydrogen bond to each other.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
Which molecule listed is heterocyclic?
A)aniline
B)phenol
C)pyridine
D)benzoic acid
E)naphthalene
A)aniline
B)phenol
C)pyridine
D)benzoic acid
E)naphthalene

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
The reaction of an amine with water is best represented by
A)R-NH2 + 2H2O
R-N2- + 2H3O+.
B)R-NH2 + H2O
R-NH- + H3O+.
C)R-NH2 + H2O
R-NH3+ + OH-.
D)R-NH2 + 2H2O
R-NH4+2 + 2OH-.
E)R-NH2 + H2O
R-N2- + M+ + H3O+.
A)R-NH2 + 2H2O

R-N2- + 2H3O+.
B)R-NH2 + H2O

R-NH- + H3O+.
C)R-NH2 + H2O

R-NH3+ + OH-.
D)R-NH2 + 2H2O

R-NH4+2 + 2OH-.
E)R-NH2 + H2O

R-N2- + M+ + H3O+.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
What is the most important chemical property of amines?
A)They are weak bases.
B)They are strong bases.
C)They are weak acids.
D)They are strong acids.
E)They are oxidizing acids.
A)They are weak bases.
B)They are strong bases.
C)They are weak acids.
D)They are strong acids.
E)They are oxidizing acids.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
The reaction that occurs between an amine and an acid is best illustrated by
A)(CH3)2NH + HCl → (CH3)2NH + Cl-.
B)(CH3)2NH + HCl → (CH3)2NH Cl- + H3O+.
C)(CH3)2NH + HCl → (CH3)2NH2 + Cl-.
D)(CH3)2NH + HCl → (CH3)2NH2+ + OH-.
E)(CH3)2NH + H2O → (CH3)2N + H3O+.
A)(CH3)2NH + HCl → (CH3)2NH + Cl-.
B)(CH3)2NH + HCl → (CH3)2NH Cl- + H3O+.
C)(CH3)2NH + HCl → (CH3)2NH2 + Cl-.
D)(CH3)2NH + HCl → (CH3)2NH2+ + OH-.
E)(CH3)2NH + H2O → (CH3)2N + H3O+.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
Which class of amines can form intermolecular hydrogen bonds?
A)1°
B)2°
C)3°
D)A and B
E)all of the above
A)1°
B)2°
C)3°
D)A and B
E)all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
Which amine has the lowest boiling point?
A)
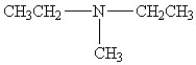
B)
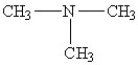
C)
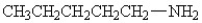
D)
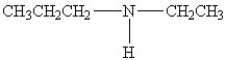
A)

B)

C)
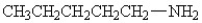
D)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
Arrange the following compounds in order of increasing boiling point.List and describe the criteria that must be considered in answering this question.
Compounds
1.CH3CH2CH2CH2OH 2.CH3CH2CH2NHCH3
3.CH3CH2CH2CH2CH3 4.CH3CH2N(CH3)CH3
5.CH3CH2CH(OH)CH3
Compounds
1.CH3CH2CH2CH2OH 2.CH3CH2CH2NHCH3
3.CH3CH2CH2CH2CH3 4.CH3CH2N(CH3)CH3
5.CH3CH2CH(OH)CH3

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Which class of amines cannot form intermolecular hydrogen bonds?
A)1°
B)2°
C)3°
D)A and B
E)all of the above
A)1°
B)2°
C)3°
D)A and B
E)all of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
Which formula best represents the form a primary amine takes in acidic solution?
A)RNH2
B)RNH-
C)RNH2-
D)RNH2+
E)RNH3+
A)RNH2
B)RNH-
C)RNH2-
D)RNH2+
E)RNH3+

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following amines would be the most soluble in water?
A)butylamine
B)propylamine
C)ethylamine
D)pentylamine
E)isopropylamine
A)butylamine
B)propylamine
C)ethylamine
D)pentylamine
E)isopropylamine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
An alkaloid is best described as any
A)basic compound obtained from a plant.
B)amide obtained from a plant.
C)basic compound obtained from either an animal or a plant.
D)diamine obtained from an animal.
E)both A and B.
A)basic compound obtained from a plant.
B)amide obtained from a plant.
C)basic compound obtained from either an animal or a plant.
D)diamine obtained from an animal.
E)both A and B.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
An alkaloid that is a chemically altered opiate known for its very addictive properties is
A)morphine.
B)codeine.
C)atropine.
D)coniine.
E)heroin.
A)morphine.
B)codeine.
C)atropine.
D)coniine.
E)heroin.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
Lemon juice can be used to remove the odor of fish on a person's hands after cleaning fish.The chemical explanation for this is that the
A)pleasant odor of the lemon juice covers the fishy odor.
B)acid in the lemon juice reacts with the odor-causing amines to form an odorless salt.
C)acid in the lemon juice increases the volatility of the odor-causing amines.
D)lemon juice dilutes the odor-causing amines.
E)lemon juice removes the bitter flavor often associated with nitrogen compounds.
A)pleasant odor of the lemon juice covers the fishy odor.
B)acid in the lemon juice reacts with the odor-causing amines to form an odorless salt.
C)acid in the lemon juice increases the volatility of the odor-causing amines.
D)lemon juice dilutes the odor-causing amines.
E)lemon juice removes the bitter flavor often associated with nitrogen compounds.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
The reaction of the pyridinium ion with water is best represented as
A)C5H5NH+ + H2O C5H5N + H3O+.
C5H5N + H3O+.
B)C5H5NH+ + H2O C5H5NH22+ + OH-.
C5H5NH22+ + OH-.
C)C5H5N + H2O C5H4N + H3O+.
C5H4N + H3O+.
D)C5H5N + H2O C5H6NH+.
C5H6NH+.
E)none of these
A)C5H5NH+ + H2O
 C5H5N + H3O+.
C5H5N + H3O+.B)C5H5NH+ + H2O
 C5H5NH22+ + OH-.
C5H5NH22+ + OH-.C)C5H5N + H2O
 C5H4N + H3O+.
C5H4N + H3O+.D)C5H5N + H2O
 C5H6NH+.
C5H6NH+.E)none of these

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
Some amine drugs are administered in the form of salts in order to make them
A)form into pills more easily.
B)taste bitter.
C)more basic.
D)more soluble in body fluids.
A)form into pills more easily.
B)taste bitter.
C)more basic.
D)more soluble in body fluids.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
All of the following are characteristics of alkaloids except
A)bitter tasting.
B)physiologically active.
C)basic.
D)toxic to humans in high doses.
E)pleasant smelling.
A)bitter tasting.
B)physiologically active.
C)basic.
D)toxic to humans in high doses.
E)pleasant smelling.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
Which type of amine will react with mineral acids to form soluble ammonium salts?
A)1°
B)2°
C)3°
D)A and B
E)A,B,and C
A)1°
B)2°
C)3°
D)A and B
E)A,B,and C

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
An alkaloid used in treating malaria is
A)quinine.
B)histamine.
C)atropine.
D)nicotine.
A)quinine.
B)histamine.
C)atropine.
D)nicotine.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The following is an example of a 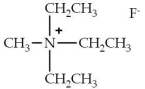
A)tertiary amine.
B)quanternary amine.
C)tertiary ammonium salt.
D)quaternary ammonium salt.
E)secondary ammonium salt.

A)tertiary amine.
B)quanternary amine.
C)tertiary ammonium salt.
D)quaternary ammonium salt.
E)secondary ammonium salt.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
Write the complete reaction for the following:
A)aniline + HBr(aq)
B)propyl amine + water
A)aniline + HBr(aq)
B)propyl amine + water

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
An alkaloid that is the major component in opium and is used to treat patients for pain after major surgeries is
A)atropine.
B)nicotine.
C)morphine.
D)reserpine.
E)caffeine.
A)atropine.
B)nicotine.
C)morphine.
D)reserpine.
E)caffeine.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
To regenerate a free amine from an ammonium salt which of the following may be used?
A)strong acid
B)an another ammonium salt
C)strong base
D)an amide
E)none of the above
A)strong acid
B)an another ammonium salt
C)strong base
D)an amide
E)none of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
Which compound is an example of an amine salt?
A)
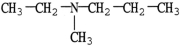
B)
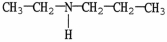
C)
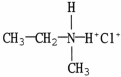
D)
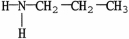
E)
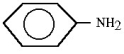
A)
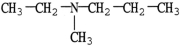
B)
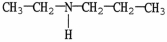
C)

D)
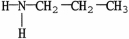
E)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
Provide the products for the following reactions:
A)
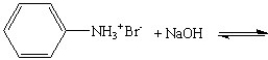
B)
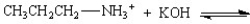
A)
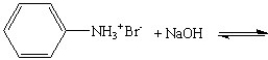
B)
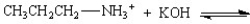

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
If methylamine reacts with hydrochloric acid,the major product will be
A)ammonium chloride.
B)dimethylammonium chloride.
C)methylammonium chloride.
D)trimethylammonium chloride.
E)methylammonium hydroxide.
A)ammonium chloride.
B)dimethylammonium chloride.
C)methylammonium chloride.
D)trimethylammonium chloride.
E)methylammonium hydroxide.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
Which compound is an example of an amine salt?
A)histamine
B)methylammonium chloride
C)thioacetamide
D)sulfanilamide
E)pyridoxine
A)histamine
B)methylammonium chloride
C)thioacetamide
D)sulfanilamide
E)pyridoxine

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
Which of these types of compounds forms salts with acids?
A)ketones
B)amines
C)alcohols
D)carboxylic esters
E)ethers
A)ketones
B)amines
C)alcohols
D)carboxylic esters
E)ethers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
All of the following are nitrogen-containing compounds found in living organisms except
A)nucleotides.
B)proteins.
C)neurotransmitters.
D)carbohydrates.
E)alkaloids.
A)nucleotides.
B)proteins.
C)neurotransmitters.
D)carbohydrates.
E)alkaloids.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
An amine that is insoluble in water can be made to dissolve by adding it to an aqueous solution of
A)HCl.
B)NaOH.
C)a different amine.
D)an amide.
E)none of the above;It can't be made water soluble.
A)HCl.
B)NaOH.
C)a different amine.
D)an amide.
E)none of the above;It can't be made water soluble.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


