Deck 9: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 9: Solutions
1
Which is not an example of a solution?
A)the mixture of gases in a SCUBA diving tank
B)a coin made from nickel and copper
C)spring water purchased at a supermarket
D)antifreeze in a car radiator
E)none of the above
A)the mixture of gases in a SCUBA diving tank
B)a coin made from nickel and copper
C)spring water purchased at a supermarket
D)antifreeze in a car radiator
E)none of the above
none of the above
2
Which of the following has the least effect on the solubility of a solid in a liquid solvent?
A)temperature
B)pressure
C)nature of the solvent
D)nature of the solute
E)concentration of solute
A)temperature
B)pressure
C)nature of the solvent
D)nature of the solute
E)concentration of solute
pressure
3
A supersaturated solution
A)contains as much solvent as it can hold.
B)contains no double bonds.
C)contains dissolved solute in equilibrium with undissolved solid.
D)will rapidly precipitate if a seed crystal is added.
E)none of the above
A)contains as much solvent as it can hold.
B)contains no double bonds.
C)contains dissolved solute in equilibrium with undissolved solid.
D)will rapidly precipitate if a seed crystal is added.
E)none of the above
will rapidly precipitate if a seed crystal is added.
4
Which homogeneous mixture is opaque and has particles large enough to be filtered?
A)colloid
B)solution
C)suspension
D)both colloids and suspensions
E)none of the above
A)colloid
B)solution
C)suspension
D)both colloids and suspensions
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
When a solid dissolves,each molecule is removed from the crystal by interaction with the solvent.This process of surrounding each ion with solvent molecules is called
A)dilution.
B)solvation.
C)electrolysis.
D)hemolysis.
E)crenation.
A)dilution.
B)solvation.
C)electrolysis.
D)hemolysis.
E)crenation.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
The dissolving process is exothermic when the energy
A)released in solvation exceeds the energy used in breaking up solute-solute and solvent-solvent interactions.
B)used in solvation exceeds the energy released in breaking up solute-solute and solvent-solvent interactions.
C)released in solvation is about the same as the energy used in breaking up solute-solute and solvent-solvent interactions.
D)used in solvation is less than the energy released in breaking up solute-solute and solvent-solvent interactions.
E)used in solvation is about the same as the energy released in breaking up solute-solute and solvent-solvent interactions.
A)released in solvation exceeds the energy used in breaking up solute-solute and solvent-solvent interactions.
B)used in solvation exceeds the energy released in breaking up solute-solute and solvent-solvent interactions.
C)released in solvation is about the same as the energy used in breaking up solute-solute and solvent-solvent interactions.
D)used in solvation is less than the energy released in breaking up solute-solute and solvent-solvent interactions.
E)used in solvation is about the same as the energy released in breaking up solute-solute and solvent-solvent interactions.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following compounds would be least soluble in water?
A)CH3NH2
B)CH3CH2OH
C)CH3COOH
D)CH3OCH3
E)CH3CH2CH2CH2CH3
A)CH3NH2
B)CH3CH2OH
C)CH3COOH
D)CH3OCH3
E)CH3CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
In a mixture of 5 mL water,10 mL alcohol,and 50 mL acetone the solvent(s)is(are)
A)acetone.
B)alcohol.
C)water.
D)acetone and alcohol.
E)alcohol and water.
A)acetone.
B)alcohol.
C)water.
D)acetone and alcohol.
E)alcohol and water.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
The number of components in a solution is
A)at least 2.
B)3.
C)4.
D)5.
E)6.
A)at least 2.
B)3.
C)4.
D)5.
E)6.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement about solubility is not true?
A)No additional solute will dissolve in a saturated solution.
B)A supersaturated solution is unstable.
C)Solubility of ionic solids increases greatly with temperature.
D)In a solution containing excess solute,an equilibrium exists between dissolved and undissolved solute.
E)The solubility of substances is measured as g solute/100 mL solvent.
A)No additional solute will dissolve in a saturated solution.
B)A supersaturated solution is unstable.
C)Solubility of ionic solids increases greatly with temperature.
D)In a solution containing excess solute,an equilibrium exists between dissolved and undissolved solute.
E)The solubility of substances is measured as g solute/100 mL solvent.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following can serve as the solvent in a solution?
A)a gas
B)a liquid
C)a solid
D)a mixture of commingled liquids
E)all of the above
A)a gas
B)a liquid
C)a solid
D)a mixture of commingled liquids
E)all of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
Water can be used to dissolve which type of compounds?
A)nonpolar compounds
B)polar compounds
C)ionic compounds
D)both A and B
E)both B and C
A)nonpolar compounds
B)polar compounds
C)ionic compounds
D)both A and B
E)both B and C

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
All of the following statements describing solutions are true except
A)making a solution involves a physical change.
B)solutions are homogeneous.
C)the particles in a solution are atomic or molecular in size.
D)solutions are colorless.
E)solutions are transparent.
A)making a solution involves a physical change.
B)solutions are homogeneous.
C)the particles in a solution are atomic or molecular in size.
D)solutions are colorless.
E)solutions are transparent.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
The exothermic step in the dissolution of NaCl in water is
A)the separation of the ions in the salt.
B)the separation of the water molecules.
C)solvation (hydration)of the ions from the salt.
D)separation of the ions in the salt and the separation of the water molecules.
A)the separation of the ions in the salt.
B)the separation of the water molecules.
C)solvation (hydration)of the ions from the salt.
D)separation of the ions in the salt and the separation of the water molecules.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the following four liquids:
Water: highly polar;H-bonding
Hexanol: slightly polar;some H-bonding
Chloroform: slightly polar;no H-bonding
Octane: non-polar;no H-bonding
Which pair of liquids is immiscible?
A)water and octane
B)water and hexanol
C)hexanol and chloroform
D)chloroform and octane
E)none of the above
Water: highly polar;H-bonding
Hexanol: slightly polar;some H-bonding
Chloroform: slightly polar;no H-bonding
Octane: non-polar;no H-bonding
Which pair of liquids is immiscible?
A)water and octane
B)water and hexanol
C)hexanol and chloroform
D)chloroform and octane
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
The term miscible is used to describe which type of solution?
A)liquid/liquid
B)liquid/solid
C)gas/liquid
D)gas/gas
E)all of the above
A)liquid/liquid
B)liquid/solid
C)gas/liquid
D)gas/gas
E)all of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements about the solubility of gases in liquids is not correct?
A)A warm can of soda fizzes more than a cold can of soda when opened because gases are less soluble at higher temperature.
B)An opened can of soda goes "flat" because the partial pressure of carbon dioxide is lower in the atmosphere than in the unopened can.
C)A solution stored under an atmosphere of pure nitrogen will contain more dissolved nitrogen than the same solution stored under air because air is only 80% nitrogen.
D)Fish kills may occur in the summertime because an inadequate amount of oxygen is dissolved in the water.
E)none of the above
A)A warm can of soda fizzes more than a cold can of soda when opened because gases are less soluble at higher temperature.
B)An opened can of soda goes "flat" because the partial pressure of carbon dioxide is lower in the atmosphere than in the unopened can.
C)A solution stored under an atmosphere of pure nitrogen will contain more dissolved nitrogen than the same solution stored under air because air is only 80% nitrogen.
D)Fish kills may occur in the summertime because an inadequate amount of oxygen is dissolved in the water.
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is miscible with CH3CH2OH?
A)CH3CH2CH3
B)CH3OH
C)CCl4
D)CH3CH3
E)CBr4
A)CH3CH2CH3
B)CH3OH
C)CCl4
D)CH3CH3
E)CBr4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement best explains the meaning of the phrase "like dissolves like"?
A)A solvent will easily dissolve a solute of similar mass.
B)A solvent and solute with similar intermolecular forces will readily form a solution.
C)The only true solutions are formed when water dissolves a non-polar solute.
D)The only true solutions are formed when water dissolves a polar solute.
E)None of these statements is correct.
A)A solvent will easily dissolve a solute of similar mass.
B)A solvent and solute with similar intermolecular forces will readily form a solution.
C)The only true solutions are formed when water dissolves a non-polar solute.
D)The only true solutions are formed when water dissolves a polar solute.
E)None of these statements is correct.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following would be immiscible with carbon tetrachloride,CCl4?
A)C6H12
B)CH3CH2OH
C)Br2
D)C3H8
E)C4H10
A)C6H12
B)CH3CH2OH
C)Br2
D)C3H8
E)C4H10

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
What is the molarity of a solution prepared by dissolving 48.0 g of NaOH in enough water to make 1.50 L of solution?
A)0.0313 M
B)0.556 M
C)0.800 M
D)1.28 M
E)32.0 M
A)0.0313 M
B)0.556 M
C)0.800 M
D)1.28 M
E)32.0 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
What is the molarity of a solution prepared by dissolving 3.50 mol NaCl in enough water to make 1.50 L of solution?
A)0.429 M
B)2.33 M
C)5.25 M
D)87.8 M
E)137 M
A)0.429 M
B)2.33 M
C)5.25 M
D)87.8 M
E)137 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the concentration of nitrogen gas in water with a nitrogen gas at a partial pressure of 0.826 atm above it.Henry's law constant for nitrogen in water is 6.8 × 10-4 mol/L-atm.
A)5.6 × 10-4 M
B)8.2 × 10-3 M
C)1.2 × 103 M
D)0.43 M
A)5.6 × 10-4 M
B)8.2 × 10-3 M
C)1.2 × 103 M
D)0.43 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Pressure has a large effect only on the solubility of ________ in liquids.
A)liquid
B)solid
C)gas
D)all of the above
E)none of the above
A)liquid
B)solid
C)gas
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
What is the % (w/v)concentration of a solution containing 8.0 grams of alcohol in 50.0 mL of solution?
A)2.0%
B)4.0%
C)8.0%
D)16%
E)50%
A)2.0%
B)4.0%
C)8.0%
D)16%
E)50%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
The solubility of gases in liquids
A)increases as temperature increases and increases as pressure increases.
B)decreases as temperature increases and increases as pressure increases.
C)decreases as temperature increases and decreases as pressure increases.
D)increases as temperature increases and decreases as pressure increases.
E)is independent of temperature and increases as pressure increases.
A)increases as temperature increases and increases as pressure increases.
B)decreases as temperature increases and increases as pressure increases.
C)decreases as temperature increases and decreases as pressure increases.
D)increases as temperature increases and decreases as pressure increases.
E)is independent of temperature and increases as pressure increases.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
In general,the solubility of ________ in water decreases as temperature increases.
A)gases
B)solids
C)liquids
D)none of these
E)all of these
A)gases
B)solids
C)liquids
D)none of these
E)all of these

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
How much NaOH is present in a 250.mL sample of a 5.00% (w/v)solution?
A)5.00 g
B)10.0 g
C)12.5 g
D)25.0 g
E)250 g
A)5.00 g
B)10.0 g
C)12.5 g
D)25.0 g
E)250 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
What is the molarity of a solution prepared by dissolving 0.750 mol CaCl2 in enough water to make 0.500 L of solution?
A)0.375 M
B)0.667 M
C)1.50 M
D)83.2 M
E)166.5 M
A)0.375 M
B)0.667 M
C)1.50 M
D)83.2 M
E)166.5 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
What is the molarity of a solution prepared by dissolving 1.25 mol of AgNO3 in enough water to make 625 mL of solution?
A)2.00 M
B)0.00200 M
C)0.500 M
D)0.0118 M
E)0.340 M
A)2.00 M
B)0.00200 M
C)0.500 M
D)0.0118 M
E)0.340 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
How many moles of HCl are present in 75.0 mL of a 0.200 M solution?
A)15.0 mol
B)2.67 mol
C)0.375 mol
D)0.275 mol
E)0.0150 mol
A)15.0 mol
B)2.67 mol
C)0.375 mol
D)0.275 mol
E)0.0150 mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
Which solution is the most concentrated? Each choice refers to the same solute and solvent.
A)2.4 g solute in 2 mL solution
B)2.4 g solute in 5 mL solution
C)20 g solute in 50 mL solution
D)30 g solute in 150 mL solution
E)50 g solute in 175 mL solution
A)2.4 g solute in 2 mL solution
B)2.4 g solute in 5 mL solution
C)20 g solute in 50 mL solution
D)30 g solute in 150 mL solution
E)50 g solute in 175 mL solution

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
The solubility of nitrogen in water exposed to the atmosphere,where the partial pressure of nitrogen is 593 mm,is 5.3 × 10-4 M.At the same temperature,what would be the solubility of pure nitrogen,at a pressure of 760 mm?
A)4.1 × 10-4 M
B)6.8 × 10-4 M
C)1500 M
D)2400 M
E)none of the above
A)4.1 × 10-4 M
B)6.8 × 10-4 M
C)1500 M
D)2400 M
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
Which solution is the least concentrated? Each choice refers to the same solute and solvent.
A)2.4 g solute in 2 mL solution
B)2.4 g solute in 5 mL solution
C)20 g solute in 50 mL solution
D)30 g solute in 150 mL solution
E)50 g solute in 175 mL solution
A)2.4 g solute in 2 mL solution
B)2.4 g solute in 5 mL solution
C)20 g solute in 50 mL solution
D)30 g solute in 150 mL solution
E)50 g solute in 175 mL solution

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
What is the molarity of a solution prepared by dissolving 10.0 g of acetone,C3H6O,in enough water to make 125 mL of solution?
A)4.65 M
B)1.38 M
C)0.0465 M
D)0.0214 M
E)0.0125 M
A)4.65 M
B)1.38 M
C)0.0465 M
D)0.0214 M
E)0.0125 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
How many grams of NaOH are needed to make 750 mL of a 2.5% (w/v)solution?
A)3.9 g
B)7.5 g
C)19 g
D)20 g
E)50 g
A)3.9 g
B)7.5 g
C)19 g
D)20 g
E)50 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
Which information is necessary to determine the molarity of a solution if the chemical formula of the solute is known?
A)only the mass of solute dissolved
B)only the volume of solvent used
C)the molar mass of both the solute and the solvent used
D)the mass of solute dissolved and the volume of solvent added
E)the mass of solute dissolved and the final volume of the solution
A)only the mass of solute dissolved
B)only the volume of solvent used
C)the molar mass of both the solute and the solvent used
D)the mass of solute dissolved and the volume of solvent added
E)the mass of solute dissolved and the final volume of the solution

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
All of the statements about molarity are correct except
A)the abbreviation is M.
B)the interpretation of the symbol is "moles of solute per mole of solvent."
C)moles = molarity × volume.
D)volume = moles/molarity.
E)the molarity of a diluted solution is less than the molarity of the original solution.
A)the abbreviation is M.
B)the interpretation of the symbol is "moles of solute per mole of solvent."
C)moles = molarity × volume.
D)volume = moles/molarity.
E)the molarity of a diluted solution is less than the molarity of the original solution.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
What is the % (w/v)concentration of a solution containing 25.0 g of solute in 400.mL of solution?
A)2.50%
B)5.00%
C)6.25%
D)12.5%
E)25.0%
A)2.50%
B)5.00%
C)6.25%
D)12.5%
E)25.0%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
How much NaCl is needed to make 50.0 mL of a 16% (w/v)solution?
A)2.0 g
B)4.0 g
C)8.0 g
D)12 g
E)16 g
A)2.0 g
B)4.0 g
C)8.0 g
D)12 g
E)16 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
How many grams of Fe(NO3)3 are present in a 20.0 mL sample of a 0.500 M solution?
A)2.14 g
B)9.68 g
C)4.84 g
D)2.42 g
E)1.21 g
A)2.14 g
B)9.68 g
C)4.84 g
D)2.42 g
E)1.21 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
How many grams of C6H12O6 are needed to prepare 2.50 L of a 5.00% (w/v)solution?
A)1.25 g
B)0.200 g
C)12.5 g
D)125 g
E)200.g
A)1.25 g
B)0.200 g
C)12.5 g
D)125 g
E)200.g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
How many grams of CaCl2 are needed to prepare 125 mL of a 1.50 M solution?
A)0.188 g
B)1.69 g
C)3.00 g
D)20.8 g
E)37.0 g
A)0.188 g
B)1.69 g
C)3.00 g
D)20.8 g
E)37.0 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
A 50.0 mL sample of a 6.0 M solution of HCl is diluted to 200.mL.What is the new concentration?
A)24.0 M
B)6.0 M
C)2.10 M
D)2.00 M
E)1.50 M
A)24.0 M
B)6.0 M
C)2.10 M
D)2.00 M
E)1.50 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the concentration in ppm of a pollutant that has been measured at 450 mg per 150 kg of sample.
A)3.0 ppm
B)6.0 ppm
C)3000 ppm
D)330 ppm
E)none of the above
A)3.0 ppm
B)6.0 ppm
C)3000 ppm
D)330 ppm
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
All of the water in a 0.200 M solution of NaCl was evaporated and 0.150 mole of NaCl was obtained.What was the original volume of the sample?
A)30.0 mL
B)333 mL
C)750.mL
D)1000 mL (1.00 × 103 mL)
E)1330 mL
A)30.0 mL
B)333 mL
C)750.mL
D)1000 mL (1.00 × 103 mL)
E)1330 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
How many grams of water are needed to prepare 250.g of a solution that is 5.00% by mass NaCl?
A)5.00 g
B)12.5 g
C)238 g
D)245 g
E)1250 g
A)5.00 g
B)12.5 g
C)238 g
D)245 g
E)1250 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Which list includes all the pieces of lab equipment needed to prepare a 0.500 M solution of NaCl from the pure salt and water?
A)buret;volumetric flask
B)buret;electronic balance
C)electronic balance;volumetric flask
D)electronic balance;graduated cylinder
E)volumetric pipet and bulb;volumetric flask
A)buret;volumetric flask
B)buret;electronic balance
C)electronic balance;volumetric flask
D)electronic balance;graduated cylinder
E)volumetric pipet and bulb;volumetric flask

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
Which procedure will produce 250 mL of a solution that is 4% by volume alcohol in water?
A)4 mL of alcohol is mixed with enough water to make 250 mL of solution.
B)10 mL of alcohol is mixed with enough water to make 250 mL of solution.
C)4 mL of alcohol is mixed with 250 mL of water.
D)10 mL of alcohol is mixed with 250 mL of water.
E)none of the above
A)4 mL of alcohol is mixed with enough water to make 250 mL of solution.
B)10 mL of alcohol is mixed with enough water to make 250 mL of solution.
C)4 mL of alcohol is mixed with 250 mL of water.
D)10 mL of alcohol is mixed with 250 mL of water.
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
How many mL of 0.105 M AgNO3 are needed for an experiment that requires 0.00510 mol of AgNO3?
A)0.536 mL
B)17.8 mL
C)18.70 mL
D)20.6 mL
E)48.6 mL
A)0.536 mL
B)17.8 mL
C)18.70 mL
D)20.6 mL
E)48.6 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
How many mL of a 5.00% (w/v)glucose solution are needed to provide 20.0 g of glucose?
A)5.00 mL
B)20.0 mL
C)4.00 mL
D)200.mL
E)400.mL
A)5.00 mL
B)20.0 mL
C)4.00 mL
D)200.mL
E)400.mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
What is the final concentration of a solution prepared by adding water to 50.0 mL of 1.5 M NaOH to make 1.00 L of solution?
A)0.075 M
B)0.030 M
C)1.5 M
D)7.5 M
E)30 M
A)0.075 M
B)0.030 M
C)1.5 M
D)7.5 M
E)30 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
Normal saline is 0.920% (w/v)NaCl in water.How many grams of NaCl are needed to prepare 15.0 L of normal saline?
A)1.38 g
B)16.3 g
C)53.8 g
D)138 g
E)807 g
A)1.38 g
B)16.3 g
C)53.8 g
D)138 g
E)807 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
How many mL of a 0.200 M HNO3 solution contains 10.0 g of HNO3?
A)2.00 mL
B)31.7 mL
C)159 mL
D)315 mL
E)793 mL
A)2.00 mL
B)31.7 mL
C)159 mL
D)315 mL
E)793 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
How many grams of FeSO4 are present in a 20.0 mL sample of a 0.500 M solution?
A)65.8 g
B)6.08 g
C)3.04 g
D)1.52 g
E)0.760 g
A)65.8 g
B)6.08 g
C)3.04 g
D)1.52 g
E)0.760 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
A 50.0 mL sample of a 12.0 M solution of HCl is diluted to 250.mL.What is the new concentration?
A)60.0 M
B)12.0 M
C)10.4 M
D)3.00 M
E)2.40 M
A)60.0 M
B)12.0 M
C)10.4 M
D)3.00 M
E)2.40 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles of solute are present in 12.0 L of 3.00 M HCl?
A)36.0 mol
B)4.00 mol
C)0.250 mol
D)3.00 mol
E)0.750 mol
A)36.0 mol
B)4.00 mol
C)0.250 mol
D)3.00 mol
E)0.750 mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
A 20.0 mL sample of CuSO4 was evaporated to dryness,leaving 0.967 g of residue.What was the molarity of the original solution?
A)48.4 M
B)0.0207 M
C)0.0484 M
D)0.303 M
E)0.433 M
A)48.4 M
B)0.0207 M
C)0.0484 M
D)0.303 M
E)0.433 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
Which list includes all the pieces of lab equipment needed to prepare 0.100 M H3PO4 from a 5.00 M solution of H3PO4?
A)buret;volumetric flask
B)buret;electronic balance
C)electronic balance;volumetric flask
D)electronic balance;graduated cylinder
E)volumetric pipet and bulb;volumetric flask
A)buret;volumetric flask
B)buret;electronic balance
C)electronic balance;volumetric flask
D)electronic balance;graduated cylinder
E)volumetric pipet and bulb;volumetric flask

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
How many mL of a 0.200 M KOH solution contains 10.0 g of KOH?
A)11.2 mL
B)50.0 mL
C)178 mL
D)281 mL
E)891 mL
A)11.2 mL
B)50.0 mL
C)178 mL
D)281 mL
E)891 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
Which solution will have the highest boiling point?
A)0.050 M KNO3
B)0.075 M CaCl2
C)0.025 M NH4NO3
D)0.020 M Na3PO4
E)0.10 M C6H12O6
A)0.050 M KNO3
B)0.075 M CaCl2
C)0.025 M NH4NO3
D)0.020 M Na3PO4
E)0.10 M C6H12O6

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
How many mL of 16 M NH3 are needed to prepare 2.00 L of a 2.00 M solution?
A)250 mL
B)125 mL
C)16 mL
D)8.0 mL
E)4.0 mL
A)250 mL
B)125 mL
C)16 mL
D)8.0 mL
E)4.0 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
Which aqueous solution will have the lowest freezing point?
A)0.60 molal sucrose
B)0.50 molal KF
C)0.60 molal glucose
D)0.24 molal FeI3
A)0.60 molal sucrose
B)0.50 molal KF
C)0.60 molal glucose
D)0.24 molal FeI3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
Considering 1.0 M solutions of each substance,which contains the largest concentration of ions?
A)K2SO4
B)FeCl3
C)NaOH
D)NH3
E)KCl
A)K2SO4
B)FeCl3
C)NaOH
D)NH3
E)KCl

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
A water solution of sodium chloride is a good conductor of electricity.Due to this property,table salt is classified as a(n)
A)weak electrolyte.
B)strong electrolyte.
C)non-electrolyte.
D)semi-electrolyte.
E)none of these.
A)weak electrolyte.
B)strong electrolyte.
C)non-electrolyte.
D)semi-electrolyte.
E)none of these.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
How many mL of 14.5 M NH3 are needed to prepare 2.00 L of a 1.00 M solution?
A)138 mL
B)69.0 mL
C)276 mL
D)29.0 mL
E)7.25 mL
A)138 mL
B)69.0 mL
C)276 mL
D)29.0 mL
E)7.25 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Considering 0.10 M solutions of each substance,which contains the smallest concentration of ions?
A)(NH4)3PO4
B)Ca(NO3)2
C)FeSO4
D)K2CO3
E)Na2SO4
A)(NH4)3PO4
B)Ca(NO3)2
C)FeSO4
D)K2CO3
E)Na2SO4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Which has the lowest boiling point?
A)0.1 M Na2SO4
B)0.1 M glucose,C6H12O6
C)0.1 M MgCl2
D)0.1 M Al(NO3)3
E)pure water
A)0.1 M Na2SO4
B)0.1 M glucose,C6H12O6
C)0.1 M MgCl2
D)0.1 M Al(NO3)3
E)pure water

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Which has the highest boiling point?
A)0.1 M Na2SO4
B)0.1 M glucose,C6H12O6
C)0.1 M MgCl2
D)0.1 M Al(NO3)3
E)pure water
A)0.1 M Na2SO4
B)0.1 M glucose,C6H12O6
C)0.1 M MgCl2
D)0.1 M Al(NO3)3
E)pure water

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
Which statement comparing solutions with pure solvents is not correct?
A)A solution containing a non-volatile solute has a lower vapor pressure than pure solvent.
B)A solution containing a non-volatile solute has a lower boiling point than pure solvent.
C)A solution containing a non-volatile solute has a lower freezing point than pure solvent.
D)A solution will have a greater mass than an equal volume of pure solvent if the solute has a molar mass greater than the solvent.
E)none of the above
A)A solution containing a non-volatile solute has a lower vapor pressure than pure solvent.
B)A solution containing a non-volatile solute has a lower boiling point than pure solvent.
C)A solution containing a non-volatile solute has a lower freezing point than pure solvent.
D)A solution will have a greater mass than an equal volume of pure solvent if the solute has a molar mass greater than the solvent.
E)none of the above

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
How many grams are contained in one equivalent of iron (III)ion,Fe+3?
A)168 g
B)55.9g
C)18.6g
D)0.0537 g
E)6.02 × 1023 g
A)168 g
B)55.9g
C)18.6g
D)0.0537 g
E)6.02 × 1023 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
What is the final concentration of a solution prepared by using 75.0 mL of 18.0 M H2SO4 to prepare 500.mL of solution?
A)8.33 × 10-3 M
B)1.20 M
C)2.70 M
D)2.35 M
E)15.7 M
A)8.33 × 10-3 M
B)1.20 M
C)2.70 M
D)2.35 M
E)15.7 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Which one of the following would act like a strong electrolyte in an aqueous solution?
A)KNO3
B)CH3OH
C)CCl4
D)NH3
A)KNO3
B)CH3OH
C)CCl4
D)NH3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
Which solution will have the lowest boiling point?
A)0.050 M KNO3
B)0.075 M CaCl2
C)0.025 M NH4NO3
D)0.020 M Na3PO4
E)0.10 M C6H12O6
A)0.050 M KNO3
B)0.075 M CaCl2
C)0.025 M NH4NO3
D)0.020 M Na3PO4
E)0.10 M C6H12O6

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
How many grams are in 10.0 mEq of Ca2+?
A)0.201 g
B)40.1 g
C)20.1 g
D)0.802 g
E)0.401 g
A)0.201 g
B)40.1 g
C)20.1 g
D)0.802 g
E)0.401 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Which substance is not an electrolyte?
A)NaCl
B)HCl
C)NH4NO3
D)KOH
E)CH4
A)NaCl
B)HCl
C)NH4NO3
D)KOH
E)CH4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
How many mL of water should be added to 50.0 mL of a 15.0 M H2SO4 solution to give a final concentration of 0.300 M?
A)950 mL
B)1000 mL
C)2450 mL
D)2500 mL
E)2550 mL
A)950 mL
B)1000 mL
C)2450 mL
D)2500 mL
E)2550 mL

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
What is the final concentration if 100 mL of water is added to 25.0 mL of 6.0 M NaCl?
A)1.2 M
B)1.5 M
C)6.0 M
D)24 M
E)30 M
A)1.2 M
B)1.5 M
C)6.0 M
D)24 M
E)30 M

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
If a normal blood sample contains 4.5 mEq/L of calcium ion,how many mg of calcium are contained in a 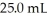
Blood sample?
A)9.0 mg
B)5.6 mg
C)2.8 mg
D)2.3 mg
E)1.4 mg
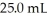
Blood sample?
A)9.0 mg
B)5.6 mg
C)2.8 mg
D)2.3 mg
E)1.4 mg

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
How many milliequivalents of chloride ion are contained in a sample that is determined to contain 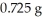
Of chloride ion?
A)48.9 mEq
B)41.0 mEq
C)25.7 mEq
D)20.5 mEq
E)10.3 mEq
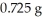
Of chloride ion?
A)48.9 mEq
B)41.0 mEq
C)25.7 mEq
D)20.5 mEq
E)10.3 mEq

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


