Deck 11: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 11: Nuclear Chemistry
1
Which type of radiation is attracted toward a negative plate?
A)α
B)β
C)γ
D)α and β
E)none of these
A)α
B)β
C)γ
D)α and β
E)none of these
α
2
Which is the best description of gamma radiation?
A)charge +2;mass of 4 amu;high penetrating power
B)charge +2;mass of 4 amu;low penetrating power
C)charge -1;mass of 0 amu;medium penetrating power
D)charge -1;mass of 0 amu;high penetrating power
E)charge 0;mass of 0 amu;high penetrating power
A)charge +2;mass of 4 amu;high penetrating power
B)charge +2;mass of 4 amu;low penetrating power
C)charge -1;mass of 0 amu;medium penetrating power
D)charge -1;mass of 0 amu;high penetrating power
E)charge 0;mass of 0 amu;high penetrating power
charge 0;mass of 0 amu;high penetrating power
3
The change of element into another by the process of nuclear decay is called
A)fission.
B)fusion.
C)transmutation.
D)translocation.
E)radiation.
A)fission.
B)fusion.
C)transmutation.
D)translocation.
E)radiation.
transmutation.
4
All of the statements about nuclear reactions are true except
A)nuclear reactions involve changes in the nucleus of an atom.
B)the rate of a nuclear reaction is increased by the addition of a catalyst.
C)a nuclear reaction is unaffected by the chemical state of the atoms involved.
D)nuclear reactions of the same element vary according to which isotope is involved.
E)energy changes in nuclear reactions are much greater than in ordinary chemical reactions.
A)nuclear reactions involve changes in the nucleus of an atom.
B)the rate of a nuclear reaction is increased by the addition of a catalyst.
C)a nuclear reaction is unaffected by the chemical state of the atoms involved.
D)nuclear reactions of the same element vary according to which isotope is involved.
E)energy changes in nuclear reactions are much greater than in ordinary chemical reactions.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
When an atom loses an alpha particle,
A)its atomic number decreases by 2 units and its mass number decreases by 4 units.
B)its atomic number increases by 1 unit but its mass number remains unchanged.
C)its mass number decreases by 1 unit but its atomic number remains unchanged.
D)neither its atomic number nor its mass number changes.
E)its atomic number increases by 2 units and its mass number increases by 4 units.
A)its atomic number decreases by 2 units and its mass number decreases by 4 units.
B)its atomic number increases by 1 unit but its mass number remains unchanged.
C)its mass number decreases by 1 unit but its atomic number remains unchanged.
D)neither its atomic number nor its mass number changes.
E)its atomic number increases by 2 units and its mass number increases by 4 units.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
Which is the best description of an alpha particle?
A)charge +2;mass of 4 amu;high penetrating power
B)charge +2;mass of 4 amu;low penetrating power
C)charge -1;mass of 0 amu;medium penetrating power
D)charge -1;mass of 0 amu;high penetrating power
E)charge 0;mass of 0 amu;high penetrating power
A)charge +2;mass of 4 amu;high penetrating power
B)charge +2;mass of 4 amu;low penetrating power
C)charge -1;mass of 0 amu;medium penetrating power
D)charge -1;mass of 0 amu;high penetrating power
E)charge 0;mass of 0 amu;high penetrating power

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these can be considered as the combination of a proton and an electron?
A)alpha particle
B)neutron
C)beta particle
D)gamma ray
E)positron
A)alpha particle
B)neutron
C)beta particle
D)gamma ray
E)positron

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is an example of a subatomic particle?
A)electron
B)proton
C)neutron
D)none of these
E)all of these
A)electron
B)proton
C)neutron
D)none of these
E)all of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
The term nucleon refers to
A)electrons belonging to an atom that undergoes nuclear decay.
B)electrons that are emitted from a nucleus in a nuclear reaction.
C)the nucleus of a specific isotope.
D)both protons and neutrons.
E)none of these
A)electrons belonging to an atom that undergoes nuclear decay.
B)electrons that are emitted from a nucleus in a nuclear reaction.
C)the nucleus of a specific isotope.
D)both protons and neutrons.
E)none of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
Which product is formed by beta emission from phosphorus-32? The atomic number of phosphorus is 15.
A)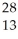 Al
Al
B)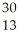 Al
Al
C)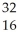 S
S
D)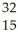 P
P
E)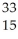 P
P
A)
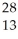 Al
AlB)
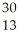 Al
AlC)
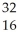 S
SD)
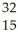 P
PE)
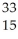 P
P
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
The emission of a particle from an unstable nucleus is called
A)mutation.
B)nuclear decay.
C)fission.
D)fusion.
E)translocation.
A)mutation.
B)nuclear decay.
C)fission.
D)fusion.
E)translocation.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
The rate of a nuclear reaction is affected by which of the following?
A)temperature
B)pressure
C)addition of a catalyst
D)none of these
E)all of these
A)temperature
B)pressure
C)addition of a catalyst
D)none of these
E)all of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
An unstable isotope generally has
A)low neutron to proton ratio.
B)high neutron to proton ratio.
C)equal number of protons and neutrons.
D)equal number of protons and electrons.
E)change in the charge of the nucleus.
A)low neutron to proton ratio.
B)high neutron to proton ratio.
C)equal number of protons and neutrons.
D)equal number of protons and electrons.
E)change in the charge of the nucleus.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
Which form of radiation is generally considered the most dangerous?
A)γ
B)β
C)α
D)positron
E)all of the above
A)γ
B)β
C)α
D)positron
E)all of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
Radioactivity is generally associated with which part of the atom?
A)nucleus
B)electrons
C)protons
D)neutrons
E)the entire atom
A)nucleus
B)electrons
C)protons
D)neutrons
E)the entire atom

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
Which type of radiation is attracted toward a positive plate?
A)α
B)β
C)γ
D)α and β
E)none of these
A)α
B)β
C)γ
D)α and β
E)none of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
Which product is formed by alpha emission from polonium-208? The atomic number of polonium is 84.
A)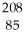 At
At
B)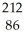 Rn
Rn
C)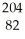 Pb
Pb
D)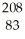 Bi
Bi
E)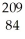 Po
Po
A)
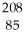 At
AtB)
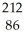 Rn
RnC)
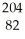 Pb
PbD)
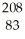 Bi
BiE)
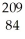 Po
Po
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
Which product is formed by alpha emission from uranium-235? The atomic number of uranium is 92.
A)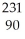 Th
Th
B)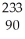 Th
Th
C)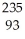 Np
Np
D)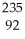 U
U
E)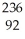 U
U
A)
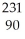 Th
ThB)
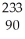 Th
ThC)
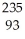 Np
NpD)
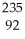 U
UE)
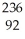 U
U
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is considered to be a radioactive isotope of hydrogen?
A).1H
B).2H
C).3H
D)all of these are unstable
E)none of these are unstable
A).1H
B).2H
C).3H
D)all of these are unstable
E)none of these are unstable

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
Which is the best description of a beta particle?
A)charge +2;mass of 4 amu;high penetrating power
B)charge +2;mass of 4 amu;low penetrating power
C)charge -1;mass of 0 amu;medium penetrating power
D)charge -1;mass of 0 amu;high penetrating power
E)charge 0;mass of 0 amu;high penetrating power
A)charge +2;mass of 4 amu;high penetrating power
B)charge +2;mass of 4 amu;low penetrating power
C)charge -1;mass of 0 amu;medium penetrating power
D)charge -1;mass of 0 amu;high penetrating power
E)charge 0;mass of 0 amu;high penetrating power

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
What other product is formed when one neutron interacts with uranium-235 to form bromine-87 and 3 neutrons?
A)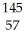 La
La
B)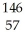 La
La
C)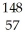 La
La
D)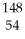 Xe
Xe
E)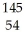 Xe
Xe
A)
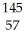 La
LaB)
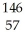 La
LaC)
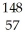 La
LaD)
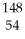 Xe
XeE)
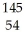 Xe
Xe
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
Which product is formed by alpha emission from gold-185? The atomic number of gold is 79.
A)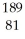 Tl
Tl
B)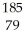 Au
Au
C)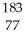 Ir
Ir
D)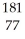 Ir
Ir
E)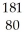 Hg
Hg
A)
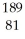 Tl
TlB)
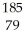 Au
AuC)
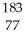 Ir
IrD)
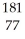 Ir
IrE)
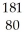 Hg
Hg
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
What is the missing reactant in the reaction shown? 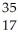 Cl + ________ →
Cl + ________ → 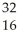 P +
P + 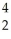 He
He
A)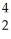 He
He
B)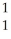 H
H
C)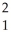 H
H
D)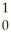 N
N
E)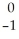 Β
Β
 Cl + ________ →
Cl + ________ →  P +
P + 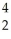 He
HeA)
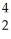 He
HeB)
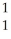 H
HC)
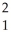 H
HD)
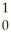 N
NE)
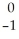 Β
Β
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
Which reaction is an example of a gamma emission?
A)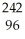 Cm →
Cm →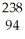 Pu +
Pu +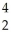 He
He
B)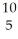 B +
B +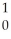 N →
N →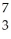 Li +
Li +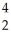 He
He
C)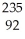 U +
U +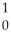 N →
N →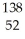 Te +
Te +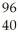 Zr + 2
Zr + 2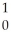 N
N
D)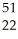 Ti →
Ti →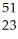 V +
V +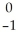 Β
Β
E)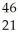 Sc →
Sc →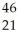 Sc + energy
Sc + energy
A)
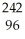 Cm →
Cm →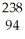 Pu +
Pu +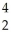 He
HeB)
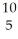 B +
B +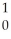 N →
N →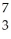 Li +
Li +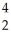 He
HeC)
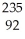 U +
U +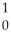 N →
N →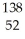 Te +
Te +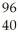 Zr + 2
Zr + 2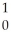 N
ND)
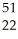 Ti →
Ti →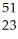 V +
V +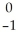 Β
ΒE)
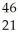 Sc →
Sc →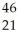 Sc + energy
Sc + energy
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
Which nuclear reaction is an example of electron capture?
A)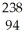 Pu +
Pu + He →
He →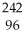 Cm
Cm
B) Na →
Na →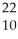 Ne +
Ne + Β
Β
C)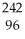 Cm →
Cm →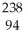 Pu +
Pu +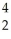 He
He
D)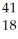 Ar +
Ar +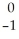 Β →
Β →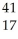 Cl
Cl
E)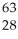 Ni →
Ni →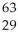 Cu +
Cu + Β
Β
A)
 Pu +
Pu +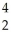 He →
He → Cm
CmB)
 Na →
Na → Ne +
Ne +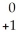 Β
ΒC)
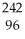 Cm →
Cm →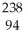 Pu +
Pu +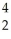 He
HeD)
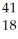 Ar +
Ar +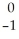 Β →
Β →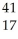 Cl
ClE)
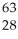 Ni →
Ni →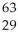 Cu +
Cu +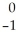 Β
Β
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
Electron capture involves the conversion of a proton to a neutron by
A)emitting an electron.
B)seizing an inner shell electron.
C)seizing an outer shell electron.
D)obtaining a gamma particle.
E)obtaining an electron from a p orbital.
A)emitting an electron.
B)seizing an inner shell electron.
C)seizing an outer shell electron.
D)obtaining a gamma particle.
E)obtaining an electron from a p orbital.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
Which nuclear reaction is an example of positron emission?
A)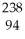 Pu +
Pu +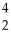 He →
He →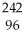 Cm
Cm
B) Na →
Na → Ne +
Ne + Β
Β
C)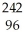 Cm →
Cm →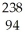 Pu +
Pu +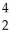 He
He
D)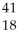 Ar +
Ar +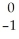 Β →
Β →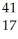 Cl
Cl
E)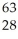 Ni →
Ni →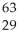 Cu +
Cu +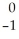 Β
Β
A)
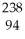 Pu +
Pu +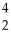 He →
He →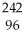 Cm
CmB)
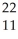 Na →
Na →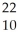 Ne +
Ne +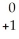 Β
ΒC)
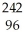 Cm →
Cm →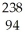 Pu +
Pu +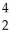 He
HeD)
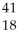 Ar +
Ar +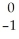 Β →
Β →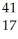 Cl
ClE)
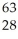 Ni →
Ni →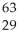 Cu +
Cu +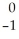 Β
Β
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
What is the product when phosphorous-30 loses a positron?
A)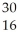 S
S
B)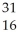 S
S
C)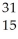 P
P
D)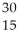 P
P
E)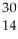 Si
Si
A)
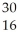 S
SB)
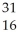 S
SC)
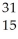 P
PD)
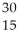 P
PE)
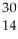 Si
Si
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
How many neutrons will be produced in the reaction shown? 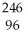 Cm +
Cm + 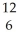 C →
C →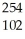 No +________
No +________ 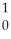 N
N
A)1
B)2
C)3
D)4
E)cannot be determined
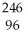 Cm +
Cm + 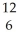 C →
C →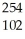 No +________
No +________ 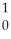 N
NA)1
B)2
C)3
D)4
E)cannot be determined

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
Which product is formed by beta emission from arsenic-75? The atomic number of arsenic is 33.
A)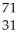 Ga
Ga
B)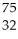 Ge
Ge
C)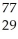 Cu
Cu
D)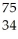 Se
Se
E)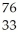 As
As
A)
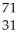 Ga
GaB)
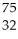 Ge
GeC)
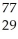 Cu
CuD)
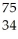 Se
SeE)
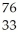 As
As
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
Which product is formed by gamma emission from technetium-99? The atomic number of technetium is 43.
A)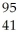 Nb
Nb
B)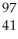 Nb
Nb
C)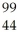 Ru
Ru
D)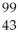 Tc
Tc
E)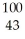 Tc
Tc
A)
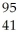 Nb
NbB)
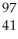 Nb
NbC)
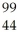 Ru
RuD)
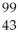 Tc
TcE)
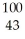 Tc
Tc
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
Write a balanced nuclear equation for a)positron emission of a calcium-38 isotope;b)formation of copper-62 by electron capture.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
Which nuclear reaction is an example of alpha emission?
A)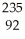 U →
U →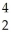 He +
He + 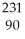 Th
Th
B)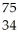 Se →
Se →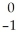 Β +
Β +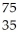 Br
Br
C)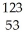 I →
I →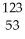 I + energy
I + energy
D)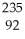 U +
U +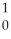 N →
N →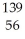 Ba +
Ba +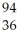 Kr + 3
Kr + 3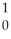 N
N
E)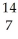 N +
N +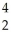 He →
He → O +
O + H
H
A)
 U →
U →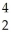 He +
He + 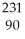 Th
ThB)
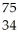 Se →
Se →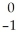 Β +
Β +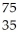 Br
BrC)
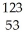 I →
I →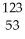 I + energy
I + energyD)
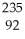 U +
U +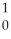 N →
N → Ba +
Ba + Kr + 3
Kr + 3 N
NE)
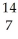 N +
N +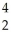 He →
He →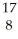 O +
O +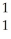 H
H
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
Which nuclear reaction is not balanced?
A)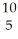 B +
B +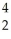 He →
He →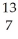 N +
N +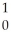 N
N
B)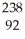 U +
U +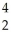 He →
He →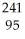 Am +
Am +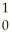 N
N
C)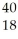 Ar +
Ar +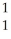 H →
H →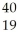 K +
K +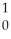 N
N
D)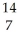 N +
N +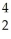 He →
He →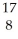 O +
O +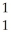 H
H
E)none of the above
A)
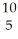 B +
B +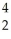 He →
He →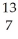 N +
N +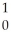 N
NB)
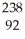 U +
U +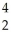 He →
He →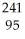 Am +
Am +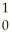 N
NC)
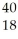 Ar +
Ar +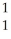 H →
H →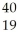 K +
K +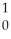 N
ND)
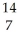 N +
N +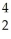 He →
He →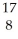 O +
O +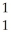 H
HE)none of the above

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
What is the missing reactant in the reaction shown?
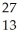 Al + ________ →
Al + ________ → 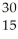 P +
P +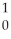 N
N
A)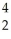 He
He
B)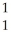 H
H
C)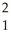 H
H
D)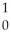 N
N
E)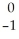 Β
Β
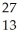 Al + ________ →
Al + ________ → 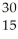 P +
P +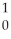 N
NA)
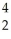 He
HeB)
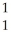 H
HC)
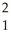 H
HD)
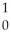 N
NE)
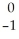 Β
Β
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
What percentage of a radioactive sample remains after four half-lives have passed?
A)0%
B)6.25%
C)12.5%
D)25%
E)50%
A)0%
B)6.25%
C)12.5%
D)25%
E)50%

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
List and discuss two criteria used to choose appropriate radioisotopes for use as tracers in medical imaging procedures.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
Which nuclear reaction is an example of beta emission?
A)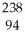 Pu +
Pu + He →
He →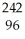 Cm
Cm
B) Na →
Na →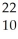 Ne +
Ne +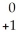 Β
Β
C)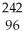 Cm →
Cm →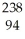 Pu +
Pu +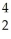 He
He
D)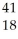 Ar +
Ar +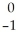 Β →
Β →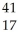 Cl
Cl
E)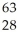 Ni →
Ni →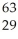 Cu +
Cu +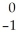 Β
Β
A)
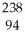 Pu +
Pu +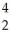 He →
He →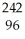 Cm
CmB)
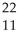 Na →
Na →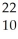 Ne +
Ne +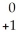 Β
ΒC)
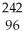 Cm →
Cm →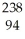 Pu +
Pu +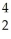 He
HeD)
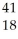 Ar +
Ar +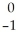 Β →
Β →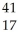 Cl
ClE)
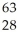 Ni →
Ni →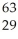 Cu +
Cu +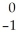 Β
Β
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Which product is formed by gamma emission from niobium-94? The atomic number of niobium is 41.
A)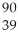 Y
Y
B)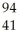 Nb
Nb
C)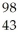 Tc
Tc
D)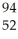 Mo
Mo
E)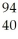 Zr
Zr
A)
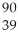 Y
YB)
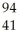 Nb
NbC)
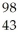 Tc
TcD)
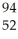 Mo
MoE)
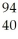 Zr
Zr
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
When a radioactive isotope decays into a nucleus which is also unstable and undergoes decay,and this process is repeated several times,the succession of reactions is called a
A)decay series.
B)fission reaction.
C)fusion reaction.
D)half-life.
E)none of these
A)decay series.
B)fission reaction.
C)fusion reaction.
D)half-life.
E)none of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
Discuss the harmful effects of ionizing radiation on the human body,including the factors affecting the degree of harm and the protective measures that can be used to minimize exposure.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is not an example of ionizing radiation?
A)X-rays
B)gamma rays
C)beta particles
D)alpha particles
E)infrared rays
A)X-rays
B)gamma rays
C)beta particles
D)alpha particles
E)infrared rays

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Ionizing radiation is dangerous to living things because
A)it causes nuclear reactions.
B)it causes thermal burns.
C)it alters the chemical structure of atoms or molecules.
D)it causes electrons to be captured by the nucleus.
E)its penetrating power varies with its source.
A)it causes nuclear reactions.
B)it causes thermal burns.
C)it alters the chemical structure of atoms or molecules.
D)it causes electrons to be captured by the nucleus.
E)its penetrating power varies with its source.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
If a technician is ordered to deliver 30.units of radiation to a patient,at what distance should the source be if it delivers 85 units for a distance of 3.5 m?
A)9.3 m
B)1.3 m
C)35 m
D)4.3 m
E)3.15 m
A)9.3 m
B)1.3 m
C)35 m
D)4.3 m
E)3.15 m

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
If 75.0 mg of potassium-42 was administered to a patient at 10 AM on Monday,how many mg will remain at 10 AM on Thursday of that same week? The half life of K-42 is 12 hours.
A)37.5 mg
B)1.17 mg
C)18.8 mg
D)9.38 mg
A)37.5 mg
B)1.17 mg
C)18.8 mg
D)9.38 mg

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
Approximately how old is a fossil that has a little more than 6% of its original radioactivity? The half-life of carbon-14 used in dating artifacts is 5700 years.
A)57,000 years
B)34,200 years
C)20,000 years
D)11,400 years
E)5700 years
A)57,000 years
B)34,200 years
C)20,000 years
D)11,400 years
E)5700 years

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
The half-life of sodium-24 is 15.0 hours.What percentage of a sample of sodium-24 remains after 75.0 hours?
A)0.00%
B)3.13%
C)6.25%
D)12.5%
E)25.0%
A)0.00%
B)3.13%
C)6.25%
D)12.5%
E)25.0%

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following has the highest ionizing power?
A)alpha
B)gamma
C)beta
D)proton
A)alpha
B)gamma
C)beta
D)proton

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following isotopes can be used to monitor spleen function?
A).99Tc
B).14C
C).3H
D).131I
E).60Co
A).99Tc
B).14C
C).3H
D).131I
E).60Co

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
In a radioactive decay series,a radioisotope decays into another radioisotope successively until a stable nucleus is produced. 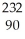 Th
Th
begins a decay series that consists of six alpha decays and four beta decays.What is the final stable isotope produced in this series?
A)plutonium-256
B)thorium-232
C)radon-220
D)lead-208
E)uranium-238
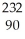 Th
Thbegins a decay series that consists of six alpha decays and four beta decays.What is the final stable isotope produced in this series?
A)plutonium-256
B)thorium-232
C)radon-220
D)lead-208
E)uranium-238

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Radioisotopes which emit alpha rays make the best diagnostic tracers.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
If the half-life of vanadium-48 is 16 days,it is true that
A)vanadium-48 is a beta emitter.
B)the decay rate would be different if the chemical environment of vanadium-48 is changed.
C)after 32 days a sample of vanadium-48 would have completely decayed.
D)after 16 days 50% of a sample of vanadium-48 would have decayed.
E)vanadium-48 would decay faster in its first half-life than in later half-lives.
A)vanadium-48 is a beta emitter.
B)the decay rate would be different if the chemical environment of vanadium-48 is changed.
C)after 32 days a sample of vanadium-48 would have completely decayed.
D)after 16 days 50% of a sample of vanadium-48 would have decayed.
E)vanadium-48 would decay faster in its first half-life than in later half-lives.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
Three applications of nuclear chemistry are medical imaging,energy production,and archaeological dating.Describe each of these applications and explain what properties of radioactive elements are used in each one.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following isotopes can be used to monitor thyroid function?
A).131I
B).14C
C).3H
D).99Tc
E).60Co
A).131I
B).14C
C).3H
D).99Tc
E).60Co

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
Approximately how old is a fossil that contains 3.13% of its original carbon-14? The half-life of carbon-14 is 5730 years.
A)2870 years
B)5730 years
C)11,500 years
D)22,900 years
E)28,700 years
A)2870 years
B)5730 years
C)11,500 years
D)22,900 years
E)28,700 years

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
Diagnostic tracers form hot spots when they are prevented from entering diseased tissue.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
The half-life of nickel-65 is 2.50 days.How much of a 100.-g sample remains after 7.50 days?
A)100.g
B)50.0 g
C)25.0 g
D)12.5 g
E)6.25 g
A)100.g
B)50.0 g
C)25.0 g
D)12.5 g
E)6.25 g

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
You obtain a new sample of cobalt-60,half-life 5.25 years,with a mass of 400.mg.How much cobalt-60 remains after 15.75 years?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
Ionizing radiation is
A)radiation that only interacts with ions.
B)the same as a proton.
C)a neutron that has acquired a charge,thus forming an ion.
D)high-energy radiation that removes electrons from atoms or molecules.
E)given off by ions and reacts with nuclei.
A)radiation that only interacts with ions.
B)the same as a proton.
C)a neutron that has acquired a charge,thus forming an ion.
D)high-energy radiation that removes electrons from atoms or molecules.
E)given off by ions and reacts with nuclei.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the properties of radioisotopes make them useful as tracers in medical or agricultural applications?
I.Their chemical behavior is the same as nonradioactive isotopes.
II.They emit various types of radiation.
III.The nuclear reaction is unaffected by the chemical state of the isotope.
A)I only
B)I and II
C)I and III
D)none of these
E)all of these
I.Their chemical behavior is the same as nonradioactive isotopes.
II.They emit various types of radiation.
III.The nuclear reaction is unaffected by the chemical state of the isotope.
A)I only
B)I and II
C)I and III
D)none of these
E)all of these

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
In nuclear fusion
A)an atomic nucleus splits into two fragments,each forming an atom of an element with a smaller atomic number than the original.
B)an atomic nucleus loses two or more alpha particles.
C)positrons and electrons combine to form gamma rays.
D)several small nuclei combine to form an atom of greater atomic number.
A)an atomic nucleus splits into two fragments,each forming an atom of an element with a smaller atomic number than the original.
B)an atomic nucleus loses two or more alpha particles.
C)positrons and electrons combine to form gamma rays.
D)several small nuclei combine to form an atom of greater atomic number.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Which reaction illustrates artificial transmutation by alpha bombardment?
A)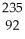 U →
U →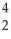 He +
He +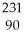 Th
Th
B)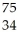 Se →
Se →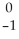 Β +
Β +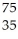 Br
Br
C)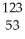 I →
I →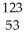 I + energy
I + energy
D)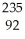 U +
U +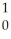 N →
N →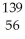 Ba +
Ba +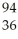 Kr + 3
Kr + 3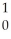 N
N
E)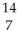 N +
N +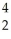 He →
He →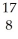 O +
O + H
H
A)
 U →
U →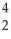 He +
He +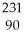 Th
ThB)
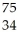 Se →
Se →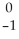 Β +
Β +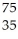 Br
BrC)
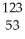 I →
I →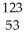 I + energy
I + energyD)
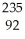 U +
U +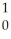 N →
N → Ba +
Ba + Kr + 3
Kr + 3 N
NE)
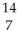 N +
N +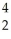 He →
He →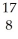 O +
O +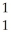 H
H
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
A rem is
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that that measures both the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that that measures both the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
The amount of a radioactive substance that undergoes 3.7 × 1010 disintegrations per second is known as a
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
The fusion reaction that supplies the energy of the sun is
A)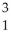 H +
H +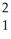 H→
H→ 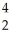 He +
He +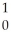 N.
N.
B)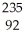 U +
U + N →
N →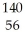 Ba +
Ba +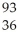 Kr + 3
Kr + 3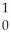 N.
N.
C)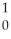 N +
N +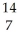 N →
N →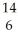 C +
C +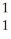 H.
H.
D)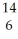 C →
C →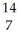 N +
N +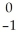 Β.
Β.
A)
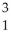 H +
H +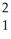 H→
H→ 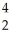 He +
He +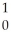 N.
N.B)
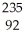 U +
U + N →
N → Ba +
Ba + Kr + 3
Kr + 3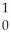 N.
N.C)
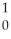 N +
N +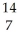 N →
N →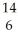 C +
C +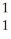 H.
H.D)
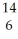 C →
C →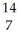 N +
N +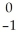 Β.
Β.
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
The SI unit for the energy absorbed is
A)becquerel.
B)gray.
C)rad.
D)sivert.
E)rem.
A)becquerel.
B)gray.
C)rad.
D)sivert.
E)rem.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
The fission of an atom of uranium (or an atom of greater atomic number)can be induced by bombarding it with
A)neutrons.
B)protons.
C)electrons.
D)positrons.
E)gamma rays.
A)neutrons.
B)protons.
C)electrons.
D)positrons.
E)gamma rays.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
The major source of radiation exposure for the general population is
A)medical sources.
B)nuclear power plants.
C)industrial activities.
D)radon in homes.
E)space.
A)medical sources.
B)nuclear power plants.
C)industrial activities.
D)radon in homes.
E)space.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
Which reaction is an example of a chain reaction?
A)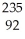 U →
U →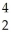 He +
He +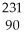 Th
Th
B)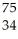 Se →
Se →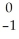 Β +
Β +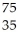 Br
Br
C)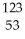 I →
I →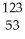 I + energy
I + energy
D)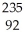 U +
U + N →
N →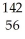 Ba +
Ba +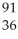 Kr + 3
Kr + 3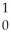 N
N
E)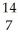 N +
N +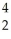 He →
He →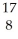 O +
O + H
H
A)
 U →
U → He +
He + Th
ThB)
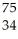 Se →
Se →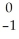 Β +
Β +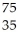 Br
BrC)
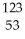 I →
I →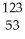 I + energy
I + energyD)
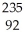 U +
U + N →
N →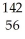 Ba +
Ba +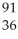 Kr + 3
Kr + 3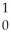 N
NE)
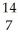 N +
N +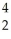 He →
He →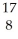 O +
O +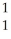 H
H
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
A curie is
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that allows both for the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that allows both for the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
A roentgen is
A)the amount of radiation dosage that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that allows both for the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.
A)the amount of radiation dosage that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that allows both for the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Which reaction illustrates artificial transmutation by bombardment with a neutron?
A)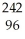 Cm →
Cm →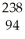 Pu +
Pu +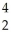 He
He
B)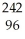 Cm +
Cm +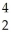 He →
He →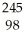 Cf +
Cf +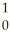 N
N
C)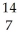 N +
N +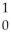 N →
N →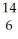 C +
C +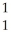 H
H
D)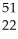 Ti →
Ti →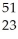 V +
V +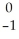 Β
Β
E)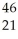 Sc →
Sc →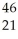 Sc + energy
Sc + energy
A)
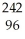 Cm →
Cm →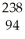 Pu +
Pu +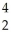 He
HeB)
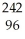 Cm +
Cm +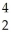 He →
He →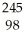 Cf +
Cf +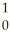 N
NC)
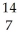 N +
N +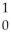 N →
N →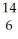 C +
C +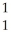 H
HD)
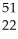 Ti →
Ti →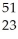 V +
V +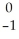 Β
ΒE)
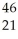 Sc →
Sc →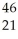 Sc + energy
Sc + energy
Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
The most important unit for biological doses of radiation is the
A)rem.
B)rad.
C)curie.
D)bozon.
E)roentgen.
A)rem.
B)rad.
C)curie.
D)bozon.
E)roentgen.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
A REM is a biological radiation measurement which is independent of the type of radiation.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
A gray is
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that measures both the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that measures both the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
A rad is
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that measures both for the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.
A)the amount of radiation that produces 2.1 × 109 units of charge in one cubic centimeter of air.
B)a unit used to measure the amount of radiation absorbed per gram of tissue.
C)a unit that measures both for the energy and the penetrating power of different types of radiation.
D)the SI unit for radiation absorbed.
E)the amount of radioactive substance that undergoes 3.7 × 1010 disintegrations per second.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
The amount of radiation that produces 2.1 × 109 units of charge in 1 cm3 of air is the
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
A unit used to measure the amount of radiation absorbed per gram of tissue is the
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
The unit of radiation exposure which allows for the energy and penetrating power of different types of radiation is the
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.
A)curie.
B)roentgen.
C)rem.
D)rad.
E)sievert.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
When a nucleus is bombarded with particles and breaks into two similarly sized nuclei plus one or more small particles,the process is called
A)fission.
B)fusion.
C)spontaneous decay.
D)induced decay.
E)mutation.
A)fission.
B)fusion.
C)spontaneous decay.
D)induced decay.
E)mutation.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck


