Deck 14: Some Compounds With Oxygen, sulfur, or a Halogen
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/97
Play
Full screen (f)
Deck 14: Some Compounds With Oxygen, sulfur, or a Halogen
1
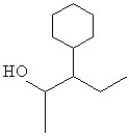
The name of the alcohol shown is
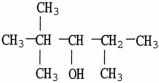
A)2,4,4-trimethyl-3-pentanol.
B)2,2,4-trimethyl-3-pentanol.
C)branched 3-octanol.
D)trimethyl-3-pentanol.
E)secondary 2,4,4-pentanol.

A)2,4,4-trimethyl-3-pentanol.
B)2,2,4-trimethyl-3-pentanol.
C)branched 3-octanol.
D)trimethyl-3-pentanol.
E)secondary 2,4,4-pentanol.
2,2,4-trimethyl-3-pentanol.
2
What is the systematic name for the following compound? 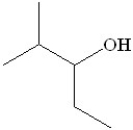
A)2-methyl-3-pentanol
B)2-methyl-3-pentenol
C)4-methyl-3-pentanol
D)3-methyl-2-pentanol

A)2-methyl-3-pentanol
B)2-methyl-3-pentenol
C)4-methyl-3-pentanol
D)3-methyl-2-pentanol
2-methyl-3-pentanol
3
Which of the following compounds will have the highest boiling point?
A)CH3CH2CH3
B)CH3CH(CH3)CH3
C)
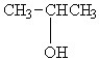
D)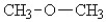
E)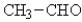
A)CH3CH2CH3
B)CH3CH(CH3)CH3
C)

D)
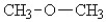
E)
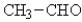

4
None of the following organic compounds is very likely to form hydrogen bonds except
A)alkanes.
B)alkenes.
C)alcohols.
D)aromatics.
E)ethers.
A)alkanes.
B)alkenes.
C)alcohols.
D)aromatics.
E)ethers.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
5
Which functional group will cause a compound to have a boiling point greater than that predicted only by molar mass?
A)ether
B)alcohol
C)alkene
D)ketone
E)alkane
A)ether
B)alcohol
C)alkene
D)ketone
E)alkane

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
6
Compounds with the -OH group attached to a saturated alkane-like carbon are known as
A)alcohols.
B)alkyl halides.
C)ethers.
D)hydroxyls.
E)phenols.
A)alcohols.
B)alkyl halides.
C)ethers.
D)hydroxyls.
E)phenols.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
7
The alcohol which contains only one carbon atom and has the common name of wood alcohol is
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
8
The IUPAC name of the alcohol shown is 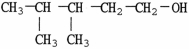
A)2,3-dimethyl-1-pentanol.
B)2,3-dimethyl-5-pentanol.
C)3,4-dimethyl-1-pentanol.
D)3,4-dimethyl-5-pentanol.
E)primary 2,3-dimethylpentanol.
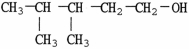
A)2,3-dimethyl-1-pentanol.
B)2,3-dimethyl-5-pentanol.
C)3,4-dimethyl-1-pentanol.
D)3,4-dimethyl-5-pentanol.
E)primary 2,3-dimethylpentanol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of the compound shown? 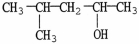
A)4-methyl-2-pentanol
B)2-methyl-4-pentanol
C)4,4-dimethyl-2-butanol
D)2,2-dimethyl-4-butanol
E)2-isohexanol
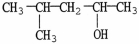
A)4-methyl-2-pentanol
B)2-methyl-4-pentanol
C)4,4-dimethyl-2-butanol
D)2,2-dimethyl-4-butanol
E)2-isohexanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is commonly known as glycerol?
A)
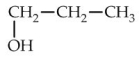
B)
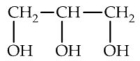
C)
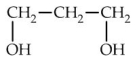
D)
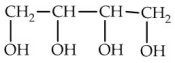
E)
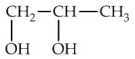
A)
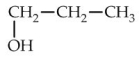
B)

C)
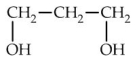
D)
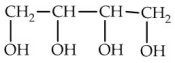
E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
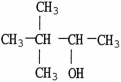
11
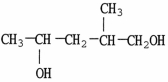
Which molecule shown is an ether?
A)
B)
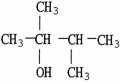
C)
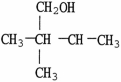
D)
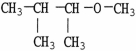
E)
A)

B)

C)

D)
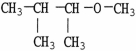
E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of the compound shown? 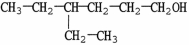
A)4-ethyl-1-hexanol
B)3-ethyl-6-hexanol
C)3-ethyl-1-hexanol
D)4,4-diethyl-1-butanol
E)isooctanol
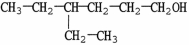
A)4-ethyl-1-hexanol
B)3-ethyl-6-hexanol
C)3-ethyl-1-hexanol
D)4,4-diethyl-1-butanol
E)isooctanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
13
What is the inorganic compound that can be considered the structural basis for alcohols and ethers? Discuss two ways in which the physical properties of alcohols and ethers are similar to properties of this compound.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
14
Alcohols,ethers,and phenols can be considered organic derivatives of the inorganic compound
A)ammonia.
B)carbon dioxide.
C)sodium hydroxide.
D)water.
E)none of these
A)ammonia.
B)carbon dioxide.
C)sodium hydroxide.
D)water.
E)none of these

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
15
Compounds with an oxygen atom bonded to two organic groups are known as
A)alcohols.
B)ethers.
C)hydroxides.
D)hydroxyls.
E)phenols.
A)alcohols.
B)ethers.
C)hydroxides.
D)hydroxyls.
E)phenols.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
16
The alcohol which contains two carbon atoms and has the common name of grain alcohol is
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
17
Rubbing alcohol is a solution of
A)ethylene glycol.
B)glycerol.
C)isopropyl alcohol.
D)ethanol.
E)methanol.
A)ethylene glycol.
B)glycerol.
C)isopropyl alcohol.
D)ethanol.
E)methanol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
18
The molecule with three carbon atoms with an -OH group on each,and used as a moisturizer is
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
19
The common name of CH3CH2OH is
A)grain alcohol.
B)wood alcohol.
C)rubbing alcohol.
D)antifreeze.
E)glycerol.
A)grain alcohol.
B)wood alcohol.
C)rubbing alcohol.
D)antifreeze.
E)glycerol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
20
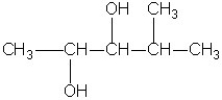
What is the IUPAC name of the compound shown? 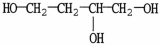
A)butanetriol
B)1,3,4-butanetriol
C)1,2,4-butanetriol
D)2-hydroxy-1,4-butanediol
E)butylene glycol
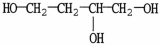
A)butanetriol
B)1,3,4-butanetriol
C)1,2,4-butanetriol
D)2-hydroxy-1,4-butanediol
E)butylene glycol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
21
All of the following properties of alcohols are affected by hydrogen bonding except
A)boiling point.
B)miscibility with water.
C)ability to dissolve polar substances.
D)molecular weight.
E)none of the above
A)boiling point.
B)miscibility with water.
C)ability to dissolve polar substances.
D)molecular weight.
E)none of the above

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
22
Compounds of the type RCH2-OH are referred to as ________ alcohols.
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of the above
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of the above

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the bond line drawing for 2-ethyl-1-methylcyclopentanol?
A)
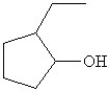
B)
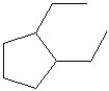
C)
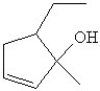
D)
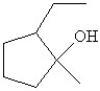
E)
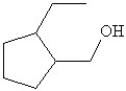
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
24
Which molecule shown is a secondary alcohol?
A)
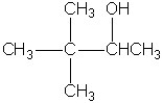
B)

C)
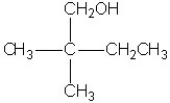
D)
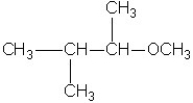
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
25
An alcohol is classified as primary,secondary or tertiary based on the
A)number of carbon atoms bonded to the carbon bearing the OH group.
B)number of carbon atoms in the molecule.
C)number of hydrogens present in the alcohol.
D)number of OH groups present in the molecule.
E)mass of the alcohol.
A)number of carbon atoms bonded to the carbon bearing the OH group.
B)number of carbon atoms in the molecule.
C)number of hydrogens present in the alcohol.
D)number of OH groups present in the molecule.
E)mass of the alcohol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound would have the highest boiling point?
A)
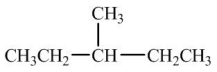
B)
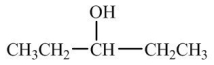
C)
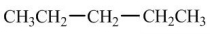
D)
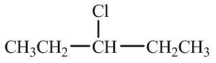
E)
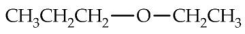
A)

B)

C)
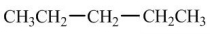
D)

E)
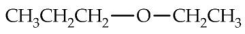

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
27
The molecule shown is a ________ alcohol because ________. 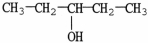
A)primary;it has one -OH group
B)primary;its -OH group is on the end of the molecule
C)secondary;the carbon bonded to the -OH group is bonded to two other carbons
D)secondary;each group bonded to the hydroxyl carbon contains two carbon atoms
E)tertiary;the -OH is bonded to the number 3 carbon

A)primary;it has one -OH group
B)primary;its -OH group is on the end of the molecule
C)secondary;the carbon bonded to the -OH group is bonded to two other carbons
D)secondary;each group bonded to the hydroxyl carbon contains two carbon atoms
E)tertiary;the -OH is bonded to the number 3 carbon

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
28
The relatively high boiling point of alcohols in relation to their molecular weights is the result of
A)covalent bonding.
B)dipolar forces.
C)hydrogen bonding.
D)ionic bonding.
E)London forces.
A)covalent bonding.
B)dipolar forces.
C)hydrogen bonding.
D)ionic bonding.
E)London forces.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
29
Which compound is the most soluble in water?
A)CH3CH2CH2CH2OH
B)CH3CH2CH2OH
C)CH3CH2CH2CH3
D)CH3CH2CH3
E)CH3CH2CH2CH2CH2CH2CH2CH3
A)CH3CH2CH2CH2OH
B)CH3CH2CH2OH
C)CH3CH2CH2CH3
D)CH3CH2CH3
E)CH3CH2CH2CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
30
Compounds of the type R3C-OH are referred to as ________ alcohols.
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of the above
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of the above

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound would you expect to have the lowest boiling point?
A)methane
B)methanol
C)dimethyl ether
D)ethanol
E)water
A)methane
B)methanol
C)dimethyl ether
D)ethanol
E)water

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
32
The common name of CH2(OH)CH2OH is
A)grain alcohol.
B)wood alcohol.
C)rubbing alcohol.
D)ethylene glycol (antifreeze).
E)glycerol.
A)grain alcohol.
B)wood alcohol.
C)rubbing alcohol.
D)ethylene glycol (antifreeze).
E)glycerol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
33
Which compound has the lowest boiling point?
A)CH3CH2CH2CH2CH2OH
B)CH3CH2CH2CH2CH3
C)CH3CH2CH2CH2OH
D)CH3CH2CH2CH3
E)CH3CH2CH2OH
A)CH3CH2CH2CH2CH2OH
B)CH3CH2CH2CH2CH3
C)CH3CH2CH2CH2OH
D)CH3CH2CH2CH3
E)CH3CH2CH2OH

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
34
Which molecule shown is a primary alcohol?
A)
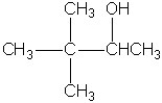
B)
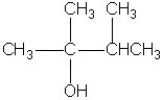
C)
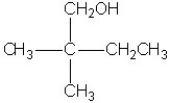
D)
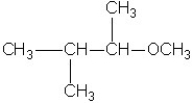
E)
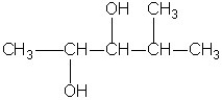
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
35
Which compound is a tertiary alcohol?
A)1-propanol
B)2-methyl-1-hexanol
C)2-methyl-2-hexanol
D)3-methyl-2-hexanol
E)3-hexanol
A)1-propanol
B)2-methyl-1-hexanol
C)2-methyl-2-hexanol
D)3-methyl-2-hexanol
E)3-hexanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
36
Which compound is the least soluble in water?
A)CH3CH2CH2CH2OH
B)CH3CH2CH2OH
C)CH3CH2CH2CH3
D)CH3CH2CH3
E)CH3CH2CH2CH2CH2CH2CH2CH3
A)CH3CH2CH2CH2OH
B)CH3CH2CH2OH
C)CH3CH2CH2CH3
D)CH3CH2CH3
E)CH3CH2CH2CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
37
Which molecule shown is a tertiary alcohol?
A)
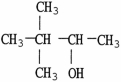
B)
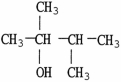
C)
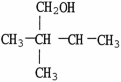
D)
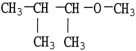
E)
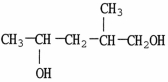
A)

B)

C)

D)
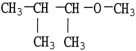
E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
38
What is the line bond drawing for 2-phenyl-3-pentanol
A)
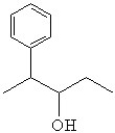
B)
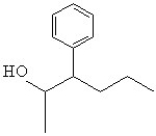
C)
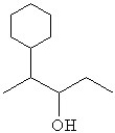
D)
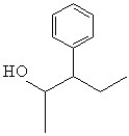
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
39
Which molecule shown is a glycol?
A)
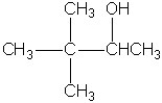
B)
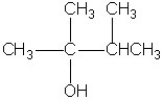
C)
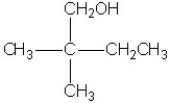
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
40
Compounds of the type R2CH-OH are referred to as ________ alcohols.
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of the above
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of the above

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is the most soluble in water?
A)HOCH2CH2CH2OH
B)CH3CH2CH2CH2OH
C)CH3CH2OCH2CH3
D)CH3CH2CH2CH3
E)CH3CH2CH2OCH3
A)HOCH2CH2CH2OH
B)CH3CH2CH2CH2OH
C)CH3CH2OCH2CH3
D)CH3CH2CH2CH3
E)CH3CH2CH2OCH3

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
42
Treatment of the molecule shown with a strong oxidizing agent will produce 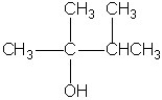
A)an aldehyde.
B)an alkene.
C)a carboxylic acid.
D)a ketone.
E)no reaction.

A)an aldehyde.
B)an alkene.
C)a carboxylic acid.
D)a ketone.
E)no reaction.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
43
Which alcohol should be used to produce 4-methyl-2-pentene by dehydration?
A)2-methyl-3-pentanol
B)4-methyl-2-pentanol
C)4-methyl-1-pentanol
D)2-methyl-1-pentanol
E)1-propanol and 2-propanol
A)2-methyl-3-pentanol
B)4-methyl-2-pentanol
C)4-methyl-1-pentanol
D)2-methyl-1-pentanol
E)1-propanol and 2-propanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
44
The product of dehydration of an alcohol is an
A)alkane.
B)alkene.
C)aromatic.
D)ether.
E)aldehyde.
A)alkane.
B)alkene.
C)aromatic.
D)ether.
E)aldehyde.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
45
The major product resulting from the dehydration of 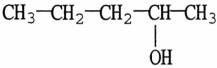
Will be
A)1-pentene.
B)2-pentene.
C)n-pentane.
D)1,2-pentanediol.
E)1,3-pentanediol.
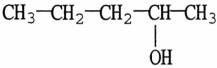
Will be
A)1-pentene.
B)2-pentene.
C)n-pentane.
D)1,2-pentanediol.
E)1,3-pentanediol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
46
Oxidation of R2CHOH will produce
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
47
The major product obtained from dehydration of 2-hexanol is
A)1-hexene.
B)2-hexene.
C)3-hexene.
D)2-hexanone.
E)2-hexanal.
A)1-hexene.
B)2-hexene.
C)3-hexene.
D)2-hexanone.
E)2-hexanal.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
48
Strong oxidation of a primary alcohol will produce
A)an aldehyde.
B)an alkene.
C)a carboxylic acid.
D)an ether.
E)a ketone.
A)an aldehyde.
B)an alkene.
C)a carboxylic acid.
D)an ether.
E)a ketone.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
49
Treatment of CH3CH2OH with an excess amount of oxidizing agent will produce
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
50
Oxidation of an alcohol group results in formation of a(an)________ group.
A)alkyl
B)aromatic
C)carbonyl
D)ether
E)hydroxyl
A)alkyl
B)aromatic
C)carbonyl
D)ether
E)hydroxyl

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
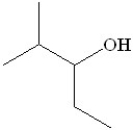
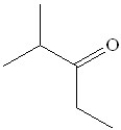
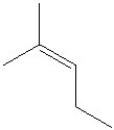
51
Which of the following would be the product of the oxidation of 2-methyl-3-pentanol?
A)
B)
C)
D)
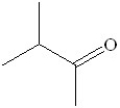
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
52
Treatment of the molecule shown with a dehydrating agent will produce 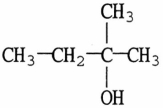
A)an aldehyde.
B)an alkene.
C)a carboxylic acid.
D)a ketone.
E)no reaction.

A)an aldehyde.
B)an alkene.
C)a carboxylic acid.
D)a ketone.
E)no reaction.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
53
Treatment of CH3CH2CH2OH with a limited amount of oxidizing agent will produce
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
54
Oxidation of a tertiary alcohol will produce
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.
A)an aldehyde.
B)a ketone.
C)an alkene.
D)a carboxylic acid.
E)no reaction.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
55
The symbol [O] written above a reaction arrow means
A)oxygen is removed from one of the reactants during the reaction.
B)the reaction consumes oxygen from the atmosphere.
C)that an oxidation reaction is occurring.
D)that a reduction reaction is occurring and oxygen is liberated.
E)none of the above
A)oxygen is removed from one of the reactants during the reaction.
B)the reaction consumes oxygen from the atmosphere.
C)that an oxidation reaction is occurring.
D)that a reduction reaction is occurring and oxygen is liberated.
E)none of the above

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following would at best be only very slightly soluble in water?
A)1-hexanol
B)2-propanol
C)1,5-pentanediol
D)1-propanol
E)2-butanol
A)1-hexanol
B)2-propanol
C)1,5-pentanediol
D)1-propanol
E)2-butanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is the most soluble in water?
A)diethyl ether
B)methanol
C)1-butanol
D)1-decanol
E)decane
A)diethyl ether
B)methanol
C)1-butanol
D)1-decanol
E)decane

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
58
Describe and explain the change in water solubility of straight-chain primary alcohols as molar mass increases.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
59
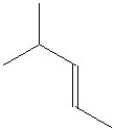
Which of the following is the major product formed from dehydration of the following alcohol? 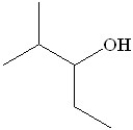
A)
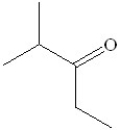
B)
C)
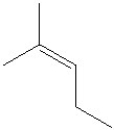
D)
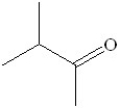

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
60
Which alcohol is most soluble in water?
A)ethanol
B)1-propanol
C)1-butanol
D)1-pentanol
E)1-hexanol
A)ethanol
B)1-propanol
C)1-butanol
D)1-pentanol
E)1-hexanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
61
Name the following: 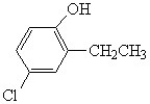
A)4-chloro-6-ethylphenol
B)3-ethyl-4-hydroxychlorobenzene
C)4-chloro-2-ethylphenol
D)meta-chloroethylphenol
E)3-chloro-6-hydroxy ethylbenzene

A)4-chloro-6-ethylphenol
B)3-ethyl-4-hydroxychlorobenzene
C)4-chloro-2-ethylphenol
D)meta-chloroethylphenol
E)3-chloro-6-hydroxy ethylbenzene

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
62
The molecule CH3CH2CH2OCH2CH3 can be classified a(n)
A)alcohol.
B)aldehyde.
C)alkane.
D)ether.
E)ketone.
A)alcohol.
B)aldehyde.
C)alkane.
D)ether.
E)ketone.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
63
Which property of thiols makes them useful as additives to natural gas?
A)flammability
B)solubility
C)odor
D)color
E)disinfectant
A)flammability
B)solubility
C)odor
D)color
E)disinfectant

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
64
Compounds with the -OH group attached to an aromatic ring are known as
A)alcohols.
B)alkyl halides.
C)ethers.
D)hydroxyls.
E)phenols.
A)alcohols.
B)alkyl halides.
C)ethers.
D)hydroxyls.
E)phenols.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
65
What is the IUPAC name of the compound shown?
CH3CH2CH2OCH3
A)1,2-etherbutane
B)methyl propyl ether
C)propyl methyl ether
D)butyl ether
E)isobutyl ether
CH3CH2CH2OCH3
A)1,2-etherbutane
B)methyl propyl ether
C)propyl methyl ether
D)butyl ether
E)isobutyl ether

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
66
Ether molecules are polar,but do not form hydrogen bonds with other ether molecules because
A)the molecules are generally too large.
B)there is no hydrogen atom bonded to the oxygen.
C)there are too many hydrogen atoms on the molecules to bond with just one oxygen atom.
D)only binary compounds form hydrogen bonds.
E)ether molecules are so reactive that they never have an opportunity to form hydrogen bonds.
A)the molecules are generally too large.
B)there is no hydrogen atom bonded to the oxygen.
C)there are too many hydrogen atoms on the molecules to bond with just one oxygen atom.
D)only binary compounds form hydrogen bonds.
E)ether molecules are so reactive that they never have an opportunity to form hydrogen bonds.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
67
All of the following are properties of ethers except
A)the molecules are polar,but do not form hydrogen bonds with other ether molecules.
B)low molecular weight ethers are flammable and evaporate easily.
C)ethers dissolve readily in water in all proportions.
D)ethers are relatively unreactive except for flammability.
E)ethers dissolve many organic compounds readily.
A)the molecules are polar,but do not form hydrogen bonds with other ether molecules.
B)low molecular weight ethers are flammable and evaporate easily.
C)ethers dissolve readily in water in all proportions.
D)ethers are relatively unreactive except for flammability.
E)ethers dissolve many organic compounds readily.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
68
Oxidation reactions are defined differently in organic chemistry than they are in inorganic chemistry.Give the definition of both and explain their similarities.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
69
Organic compounds which are sulfur analogs of alcohols are referred to as
A)sulfuric alcohols.
B)disulfides
C)halides.
D)thiols.
E)carbonyls.
A)sulfuric alcohols.
B)disulfides
C)halides.
D)thiols.
E)carbonyls.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following alcohols would not be able to be oxidized with any type of oxidizing agent?
A)
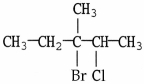
B)
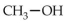
C)
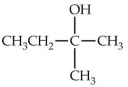
D)
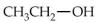
E)All compounds will react.
A)

B)
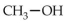
C)

D)
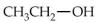
E)All compounds will react.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
71
What is the IUPAC name of the compound shown? 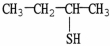
A)2-thiobutane
B)3-thiobutanol
C)2-butanethiol
D)1-methyl-1-thiopropane
E)1-methyl-1-propanethiol
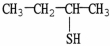
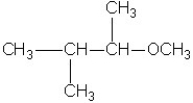
A)2-thiobutane
B)3-thiobutanol
C)2-butanethiol
D)1-methyl-1-thiopropane
E)1-methyl-1-propanethiol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
72
What is the systematic name of the following compound? 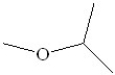
A)2-methoxy propane
B)2-ethoxy propane
C)ethyl methyl ether
D)diethyl ether

A)2-methoxy propane
B)2-ethoxy propane
C)ethyl methyl ether
D)diethyl ether

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
73
The most characteristic feature of thiols is
A)odor.
B)solubility in water.
C)reactivity with water.
D)boiling point.
E)color.
A)odor.
B)solubility in water.
C)reactivity with water.
D)boiling point.
E)color.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
74
The simplest aromatic alcohol,recognized by its strong medicinal odor and used as a disinfectant is
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.
A)ethanol.
B)glycerol.
C)glycol.
D)methanol.
E)phenol.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
75
Which molecule would be the most acidic?
A)dimethyl ether
B)ethanol
C)phenol
D)water
E)1-hexanol
A)dimethyl ether
B)ethanol
C)phenol
D)water
E)1-hexanol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
76
Which compound is sometimes called carbolic acid?
A)ethanol
B)methanol
C)glycerol
D)ether
E)phenol
A)ethanol
B)methanol
C)glycerol
D)ether
E)phenol

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
77
When phenol acts as an acid,a ________ ion is produced.
A)phenyl
B)benzyl
C)phenolate
D)phenolic
E)phenoxide
A)phenyl
B)benzyl
C)phenolate
D)phenolic
E)phenoxide

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
78
All of the following can be classified as alkyl halides except
A)styrene,the monomer used to make foam coffee cups.
B)chloroform,an anesthetic.
C)iodoform,an disinfectant.
D)methyl bromide,an insecticide.
E)CFC's used as refrigerants.
A)styrene,the monomer used to make foam coffee cups.
B)chloroform,an anesthetic.
C)iodoform,an disinfectant.
D)methyl bromide,an insecticide.
E)CFC's used as refrigerants.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
79
When a thiol is oxidized the product is
A)an aldehyde.
B)a ketone.
C)sulfuric acid.
D)a disulfide.
E)an alkene.
A)an aldehyde.
B)a ketone.
C)sulfuric acid.
D)a disulfide.
E)an alkene.

Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck
80
Which molecule would be considered a derivative of phenol?
A)
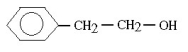
B)
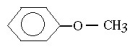
C)
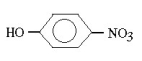
D)
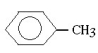
E)
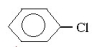
A)
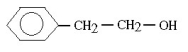
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 97 flashcards in this deck.
Unlock Deck
k this deck


