Deck 13: Alkenes, alkynes, and Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 13: Alkenes, alkynes, and Aromatic Compounds
1
Which of the following is a correct line bond structure for 2,5- dimethyl-3-hexyne?
A)
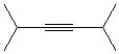
B)
C)
D)
E)
A)

B)

C)

D)

E)


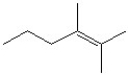
2
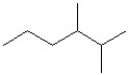
What is the IUPAC name of the molecule shown? 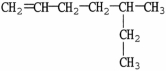
A)3-methyl-6-heptene
B)5-methyl-1-heptene
C)5-ethyl-1-hexene
D)2-ethyl-5-hexene
E)octene
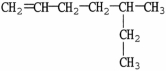
A)3-methyl-6-heptene
B)5-methyl-1-heptene
C)5-ethyl-1-hexene
D)2-ethyl-5-hexene
E)octene
5-methyl-1-heptene
3
What is the IUPAC name of the molecule shown? 
A)2-methyl-3-methyl-2-hexene
B)dimethylhexene
C)2,3-dimethyl-2-hexene
D)octene
E)1,1,2-trimethyl-1-pentene
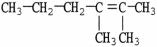
A)2-methyl-3-methyl-2-hexene
B)dimethylhexene
C)2,3-dimethyl-2-hexene
D)octene
E)1,1,2-trimethyl-1-pentene
2,3-dimethyl-2-hexene
4
What is the IUPAC name of the molecule shown?
CH2=CH-CH=CH2
A)diethylene
B)1,1-butadiene
C)1,2-butadiene
D)1,3-butadiene
E)1,4-butadiene
CH2=CH-CH=CH2
A)diethylene
B)1,1-butadiene
C)1,2-butadiene
D)1,3-butadiene
E)1,4-butadiene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds is a saturated hydrocarbon?
A)acetylene
B)benzene
C)1,3-butadiene
D)ethylene
E)hexane
A)acetylene
B)benzene
C)1,3-butadiene
D)ethylene
E)hexane

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
On the basis of the number of carbon-hydrogen bonds,all of the following families of compounds can be considered unsaturated except
A)alkanes.
B)alkenes.
C)alkynes.
D)arenes.
E)none of the above
A)alkanes.
B)alkenes.
C)alkynes.
D)arenes.
E)none of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
The cause of cis-trans isomerism is
A)stability of the double bond.
B)lack of rotation of the double bond.
C)strength of the double bond.
D)short length of the double bond.
E)vibration of the double bond.
A)stability of the double bond.
B)lack of rotation of the double bond.
C)strength of the double bond.
D)short length of the double bond.
E)vibration of the double bond.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
Which molecule represents 4-ethyl-2-hexyne?
A)(CH3CH2)2CHC≡CCH(CH2CH3)2
B)(CH3CH2)2CHC≡CCH3
C)CH3CH2CH2C≡CCH3
D)CH3CH2CH2CH2C≡CCH3
E)CH3CH2C≡CCH2CH2CH3
A)(CH3CH2)2CHC≡CCH(CH2CH3)2
B)(CH3CH2)2CHC≡CCH3
C)CH3CH2CH2C≡CCH3
D)CH3CH2CH2CH2C≡CCH3
E)CH3CH2C≡CCH2CH2CH3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of the molecule shown? 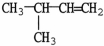
A)isopentene
B)3-methyl-1-butene
C)3-methyl-1,2-butene
D)2-methyl-3-butene
E)1,1-dimethyl-2-propene
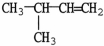
A)isopentene
B)3-methyl-1-butene
C)3-methyl-1,2-butene
D)2-methyl-3-butene
E)1,1-dimethyl-2-propene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
Draw both the condensed and line bond structures for
A)1,3,3-trimethyl cyclohexene
B)6-ethyl-3-methyl-4-octyne
A)1,3,3-trimethyl cyclohexene
B)6-ethyl-3-methyl-4-octyne

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
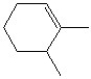
11
The name of the molecule shown is 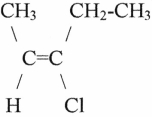
A)cis-3-chloro-2-pentene.
B)trans-3-chloro-2-pentene.
C)cis-3-chloro-3-pentene.
D)trans-3-chloro-3-pentene.
E)monochloro-2-cis-pentene.

A)cis-3-chloro-2-pentene.
B)trans-3-chloro-2-pentene.
C)cis-3-chloro-3-pentene.
D)trans-3-chloro-3-pentene.
E)monochloro-2-cis-pentene.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds contains an alkyne functional group?
A)CH3CH2CH2CH2CH3
B)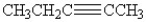
C)CH3CH(CH3)CH2CH2CH3
D)CH3CCCH2CH3
E)both B and D
A)CH3CH2CH2CH2CH3
B)
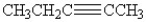
C)CH3CH(CH3)CH2CH2CH3
D)CH3CCCH2CH3
E)both B and D

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds contains an alkene functional group?
A)CH3CH2CH2CH2CH3
B)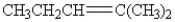
C)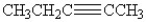
D)CH3CH(CH3)CH2CH2CH3
E)CH3CCCH2CH3
A)CH3CH2CH2CH2CH3
B)
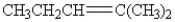
C)
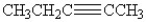
D)CH3CH(CH3)CH2CH2CH3
E)CH3CCCH2CH3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is a correct description of the difference between a saturated and an unsaturated hydrocarbon?
A)Saturated hydrocarbons are essentially water insoluble while unsaturated hydrocarbons are water soluble.
B)Unsaturated hydrocarbons are flammable but saturated hydrocarbons are not.
C)Saturated hydrocarbons do not contain multiple bonds between carbons but unsaturated hydrocarbons do contain multiple bonds.
D)Saturated hydrocarbons are composed of carbon and hydrogen but unsaturated hydrocarbons include atoms other than carbon and hydrogen.
A)Saturated hydrocarbons are essentially water insoluble while unsaturated hydrocarbons are water soluble.
B)Unsaturated hydrocarbons are flammable but saturated hydrocarbons are not.
C)Saturated hydrocarbons do not contain multiple bonds between carbons but unsaturated hydrocarbons do contain multiple bonds.
D)Saturated hydrocarbons are composed of carbon and hydrogen but unsaturated hydrocarbons include atoms other than carbon and hydrogen.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
In organic chemistry,the term unsaturated means a molecule
A)that has the maximum number of carbon-hydrogen bonds possible.
B)with a specific six-membered ring structure.
C)that contains one or more multiple bonds between carbon atoms.
D)that can react by taking up one or more water molecules.
E)that is formed from many smaller molecules.
A)that has the maximum number of carbon-hydrogen bonds possible.
B)with a specific six-membered ring structure.
C)that contains one or more multiple bonds between carbon atoms.
D)that can react by taking up one or more water molecules.
E)that is formed from many smaller molecules.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
Ethylene and acetylene are the common names for the molecules ________ and ________,respectively.
A)C2H6;C3H8
B)C2H4;C2H6
C)C2H2;C2H6
D)C2H4;C3H6
E)C2H4;C2H2
A)C2H6;C3H8
B)C2H4;C2H6
C)C2H2;C2H6
D)C2H4;C3H6
E)C2H4;C2H2

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
This question has three parts:
A) Sketch the carbon skeleton of 3-ethyl-2,5-hexadiene.
B) Explain why this name is not correct.
C) Give the correct name and molecular formula of the compound with the carbon skeleton you drew.
A) Sketch the carbon skeleton of 3-ethyl-2,5-hexadiene.
B) Explain why this name is not correct.
C) Give the correct name and molecular formula of the compound with the carbon skeleton you drew.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
Cis-trans isomerism occurs when
A)a branched alkane has a halogen added to two adjacent carbon atoms.
B)an alkene is hydrated according to Markovnikov's Rule.
C)the carbons in an alkene double bond each have two different substituent groups.
D)the carbons in the para position of an aromatic have the same substituent groups.
E)hydrogen is added to both of the carbon atoms in a double bond.
A)a branched alkane has a halogen added to two adjacent carbon atoms.
B)an alkene is hydrated according to Markovnikov's Rule.
C)the carbons in an alkene double bond each have two different substituent groups.
D)the carbons in the para position of an aromatic have the same substituent groups.
E)hydrogen is added to both of the carbon atoms in a double bond.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
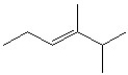
19
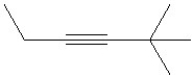
Which of the following is a correct line bond structure for 2,3-dimethyl-3-hexene?
A)
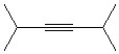
B)
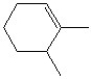
C)
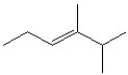
D)
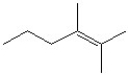
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
This question has three parts:
A) Sketch the carbon skeleton of 2,5-hexadiene.
B) Explain why this name is not correct.
C) Give the correct name and molecular formula of the compound with the carbon skeleton you drew.
A) Sketch the carbon skeleton of 2,5-hexadiene.
B) Explain why this name is not correct.
C) Give the correct name and molecular formula of the compound with the carbon skeleton you drew.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
A rearrangement reaction can best be described as a reaction in which
A)a hydrocarbon reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.
A)a hydrocarbon reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
All of the following are examples of addition reactions of alkenes except
A)bromination.
B)chlorination.
C)hydration.
D)hydrogenation.
E)oxidation.
A)bromination.
B)chlorination.
C)hydration.
D)hydrogenation.
E)oxidation.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
Alkenes and simple aromatics are similar in all of the following properties except
A)solubility in non-polar solvents.
B)insolubility in water.
C)flammability.
D)nonpolarity.
E)lack of toxicity.
A)solubility in non-polar solvents.
B)insolubility in water.
C)flammability.
D)nonpolarity.
E)lack of toxicity.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a correct line angle drawing for cis-3-heptene?
A)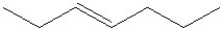
B)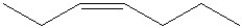
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
Which molecule can have cis-trans isomers?
A)CH3CH=C(CH3)2
B)(CH3)2C=CHCH3
C)(CH3)2C=C(CH3)2
D)CH3CH=CHCl
E)CH3CH=CCl2
A)CH3CH=C(CH3)2
B)(CH3)2C=CHCH3
C)(CH3)2C=C(CH3)2
D)CH3CH=CHCl
E)CH3CH=CCl2

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
The term used to describe the geometry of a carbon atom involved in a triple bond is
A)distorted tetrahedral.
B)linear.
C)perpendicular.
D)tetrahedral.
E)trigonal planar.
A)distorted tetrahedral.
B)linear.
C)perpendicular.
D)tetrahedral.
E)trigonal planar.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
An addition reaction can best be described as a reaction in which
A)a hydrogen reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.
A)a hydrogen reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
The bond angle about a carbon atom involved in a triple bond is
A)90°.
B)105°.
C)109.5°.
D)120°.
E)180°.
A)90°.
B)105°.
C)109.5°.
D)120°.
E)180°.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
A substitution reaction can best be described as a reaction in which
A)a hydrocarbon reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.
A)a hydrocarbon reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name of the compound shown? 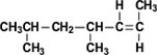
A)trans-4,6-dimethyl-2-heptene
B)cis-4,6-dimethyl-2-heptene
C)trans-2-nonene
D)cis-2-nonene
E)trans-2,4-dimethyl-5-heptene

A)trans-4,6-dimethyl-2-heptene
B)cis-4,6-dimethyl-2-heptene
C)trans-2-nonene
D)cis-2-nonene
E)trans-2,4-dimethyl-5-heptene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
The term used to describe the geometry of a carbon atom involved in a double bond is
A)distorted tetrahedral.
B)linear.
C)perpendicular.
D)tetrahedral.
E)trigonal planar.
A)distorted tetrahedral.
B)linear.
C)perpendicular.
D)tetrahedral.
E)trigonal planar.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
An elimination reaction can best be described as a reaction in which
A)a hydrocarbon reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.
A)a hydrocarbon reacts with oxygen to produce CO2,H2O,and energy.
B)two reactants combine to form one new product with no extra atoms.
C)a single reactant splits into two products.
D)two reactants exchange atoms to give two new products.
E)a single reactant undergoes reorganization of its chemical bonds,producing an isomer of the reactant.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
All of the following are general properties of alkenes except
A)soluble in non-polar (organic)solvents.
B)low boiling points.
C)flammable.
D)less reactive than the corresponding alkanes.
E)may exist as cis-trans isomers.
A)soluble in non-polar (organic)solvents.
B)low boiling points.
C)flammable.
D)less reactive than the corresponding alkanes.
E)may exist as cis-trans isomers.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
When an alkene undergoes hydrogenation,the product is an
A)alkane.
B)alkene.
C)alkyne.
D)aromatic.
E)alcohol.
A)alkane.
B)alkene.
C)alkyne.
D)aromatic.
E)alcohol.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
The name of the molecule shown is 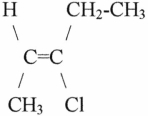
A)cis-3-chloro-2-pentene.
B)trans-3-chloro-2-pentene.
C)cis-3-chloro-3-pentene.
D)trans-3-chloro-3-pentene.
E)monochloro-2-cis-pentene.

A)cis-3-chloro-2-pentene.
B)trans-3-chloro-2-pentene.
C)cis-3-chloro-3-pentene.
D)trans-3-chloro-3-pentene.
E)monochloro-2-cis-pentene.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
The bond angle about a carbon atom involved in a double bond is
A)90°.
B)105°.
C)109.5°.
D)120°.
E)180°.
A)90°.
B)105°.
C)109.5°.
D)120°.
E)180°.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
When an alkene undergoes a hydration reaction the product is an
A)alkane.
B)alkyne.
C)aromatic.
D)alcohol.
E)ether.
A)alkane.
B)alkyne.
C)aromatic.
D)alcohol.
E)ether.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Alkanes and alkenes are similar in all of the following properties except
A)solubility in non-polar solvents.
B)insolubility in water.
C)flammability.
D)reactivity.
E)lack of toxicity.
A)solubility in non-polar solvents.
B)insolubility in water.
C)flammability.
D)reactivity.
E)lack of toxicity.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Chemical reactions involving double bonds are generally referred to as ________ reactions.
A)substitution
B)addition
C)oxidation
D)reduction
E)combustion
A)substitution
B)addition
C)oxidation
D)reduction
E)combustion

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following alkenes can exhibit cis-trans isomerism?
A)1,1-dibromobutene
B)1-pentene
C)2-methyl-2-octene
D)3-octene
E)none of these
A)1,1-dibromobutene
B)1-pentene
C)2-methyl-2-octene
D)3-octene
E)none of these

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
The monomer unit used to produce polypropylene is
A)CH2=CH2.
B)CH2=CHCH3.
C)CH3CH2CH2CH3.
D)CH2=CHCH2Cl.
E)CHCl=CH2.
A)CH2=CH2.
B)CH2=CHCH3.
C)CH3CH2CH2CH3.
D)CH2=CHCH2Cl.
E)CHCl=CH2.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
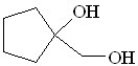
Predict the product of the following reaction: 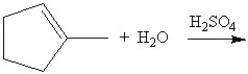
A)
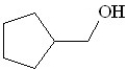
B)
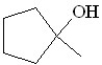
C)
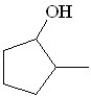
D)
E)
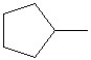
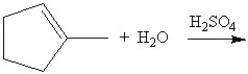
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
According to Markovnikov's rule,when HCl reacts with the molecule shown,which product will result?
(CH3)2C=CHCH3 + HCl → ?????
A)(CH3)2CHCHClCH3
B)(CH3)2CClCH2CH3
C)(CH3)2CClCHClCH3
D)(CH3)2CHCH2CH2Cl
E)Cl2CHCHClCH3
(CH3)2C=CHCH3 + HCl → ?????
A)(CH3)2CHCHClCH3
B)(CH3)2CClCH2CH3
C)(CH3)2CClCHClCH3
D)(CH3)2CHCH2CH2Cl
E)Cl2CHCHClCH3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following reagents could be used to convert 2-butene to butane?
A)Cl2
B)HCl
C)H2,Pd
D)H2O
E)H2SO4
A)Cl2
B)HCl
C)H2,Pd
D)H2O
E)H2SO4

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
Which reactant should be used to convert propene to 2-chloropropane?
A)Cl2
B)H2
C)NaCl
D)HCl
E)BrCl
A)Cl2
B)H2
C)NaCl
D)HCl
E)BrCl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
Predict the product of the following reaction: 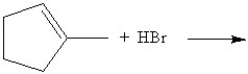
A)
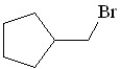
B)
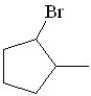
C)
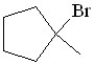
D)
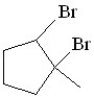
E)
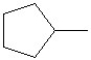
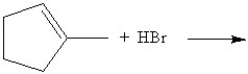
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
Which reactant should be used to convert propene to 1,2-dichloropropane?
A)Cl2
B)H2
C)NaCl
D)HCl
E)BrCl
A)Cl2
B)H2
C)NaCl
D)HCl
E)BrCl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
The concept that explains the properties of aromatic compounds based on a structure that is an average among two possible structures is
A)double bonding.
B)oxidation.
C)resonance.
D)cis-trans isomerism.
E)polymerization.
A)double bonding.
B)oxidation.
C)resonance.
D)cis-trans isomerism.
E)polymerization.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
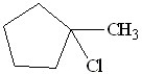
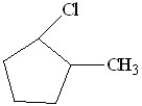
What will be the major product of the following reaction? 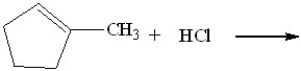
A)
B)
C)
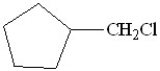
D)
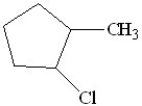
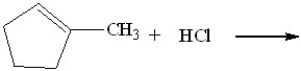
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
In the addition of HX to a double bond,the hydrogen goes to the carbon that already has more hydrogens.This is a statement of
A)the double bond rule.
B)LeChatelier's principle.
C)Markovnikov's rule.
D)the rule of "less is better."
E)Zatseff's rule.
A)the double bond rule.
B)LeChatelier's principle.
C)Markovnikov's rule.
D)the rule of "less is better."
E)Zatseff's rule.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
The commonly accepted mechanism for explaining alkene reactions involves formation of
A)carbocations.
B)carbanions.
C)carbon atoms with 10 electrons.
D)carbon atoms with four electrons.
E)carbon atoms which have lost all their electrons.
A)carbocations.
B)carbanions.
C)carbon atoms with 10 electrons.
D)carbon atoms with four electrons.
E)carbon atoms which have lost all their electrons.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
When 2-butene reacts completely with bromine,the product is
A)2-bromobutane.
B)3-bromobutane.
C)1,2-dibromobutane.
D)1,3-dibromobutane.
E)2,3-dibromobutane.
A)2-bromobutane.
B)3-bromobutane.
C)1,2-dibromobutane.
D)1,3-dibromobutane.
E)2,3-dibromobutane.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
The addition of HF to 2-butene produces
A)2-fluorobutane.
B)1-fluorobutane.
C)1,2-difluorobutane.
D)2,3-difluorobutane.
E)The reaction doesn't occur.
A)2-fluorobutane.
B)1-fluorobutane.
C)1,2-difluorobutane.
D)2,3-difluorobutane.
E)The reaction doesn't occur.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
The starting material for polymerization reactions is a(an)
A)alkane.
B)catalyst.
C)isomer.
D)monomer.
E)dimer.
A)alkane.
B)catalyst.
C)isomer.
D)monomer.
E)dimer.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
What is the name of the product when 1-pentene reacts with Cl2?
A)1,1-dichloropentane
B)1,2-dichloropentane
C)2,2-dichloropentane
D)2,3-dichloropentane
A)1,1-dichloropentane
B)1,2-dichloropentane
C)2,2-dichloropentane
D)2,3-dichloropentane

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
Markovnikov's Rule refers to the
A)orientation an unsymmetrical reagent will take when added to an unsymmetrical alkene.
B)temperature difference observed in the boiling points of cis and trans alkenes.
C)rate of hydrogen addition to an alkene with alkyl group substituents.
D)color of a molecule containing multiple double bonds.
E)ideal bond angle between substituents on a double bond.
A)orientation an unsymmetrical reagent will take when added to an unsymmetrical alkene.
B)temperature difference observed in the boiling points of cis and trans alkenes.
C)rate of hydrogen addition to an alkene with alkyl group substituents.
D)color of a molecule containing multiple double bonds.
E)ideal bond angle between substituents on a double bond.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
The monomer used to make the polymer Teflon is
A)CH2=CF2.
B)CF2=CF2.
C)CH2=CHCl.
D)CH2=CHCN.
E)CH2=CH-C6H5.
A)CH2=CF2.
B)CF2=CF2.
C)CH2=CHCl.
D)CH2=CHCN.
E)CH2=CH-C6H5.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
The name of the polymer formed from CH2=CH2 is
A)polyethylene.
B)polypropylene.
C)polystyrene.
D)polyvinyl chloride.
E)none of the above
A)polyethylene.
B)polypropylene.
C)polystyrene.
D)polyvinyl chloride.
E)none of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following reactions involves addition of two different elements to an alkene?
A)bromination
B)chlorination
C)hydrohalogenation
D)hydrogenation
E)none of the above
A)bromination
B)chlorination
C)hydrohalogenation
D)hydrogenation
E)none of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
The monomer used to make the polymer polyvinyl chloride is
A)CH2=CF2.
B)CF2=CF2.
C)CH2=CHCl.
D)CH2=CHCN.
E)CH2=CH-C6H5.
A)CH2=CF2.
B)CF2=CF2.
C)CH2=CHCl.
D)CH2=CHCN.
E)CH2=CH-C6H5.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is not the common name of an aromatic compound?
A)xylene
B)phenol
C)acetone
D)toluene
E)aniline
A)xylene
B)phenol
C)acetone
D)toluene
E)aniline

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Another name for o-nitrotoluene is
A)1-nitrotoluene.
B)2-nitrotoluene.
C)3-nitrotoluene.
D)4-nitrotoluene.
A)1-nitrotoluene.
B)2-nitrotoluene.
C)3-nitrotoluene.
D)4-nitrotoluene.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Which phrase most accurately describes the structure common to all aromatic compounds?
A)a six-membered ring with 3 double and 3 single bonds
B)a ring described as 1,3,5-hexatriene
C)identical bonds between all 6 carbon atoms,with 6 electrons moving freely
D)a six-membered ring with easily broken carbon-carbon bonds
E)none of the above
A)a six-membered ring with 3 double and 3 single bonds
B)a ring described as 1,3,5-hexatriene
C)identical bonds between all 6 carbon atoms,with 6 electrons moving freely
D)a six-membered ring with easily broken carbon-carbon bonds
E)none of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Using systematic names,the structure shown could be called 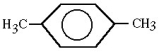
A)1,2-dimethylbenzene.
B)1,3-dimethylbenzene.
C)para-dimethylbenzene.
D)ortho-dimethylbenzene.
E)meta-dimethylbenzene.

A)1,2-dimethylbenzene.
B)1,3-dimethylbenzene.
C)para-dimethylbenzene.
D)ortho-dimethylbenzene.
E)meta-dimethylbenzene.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
What reagent is used in the nitration of benzene?
A)HNO3,H2SO4
B)FeBr3,Br2
C)HNO3
D)H2SO4
E) H2,Pd
A)HNO3,H2SO4
B)FeBr3,Br2
C)HNO3
D)H2SO4
E) H2,Pd

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
Match the following.
unsaturated
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds
unsaturated
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
The structure shown is 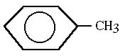
A)meta-xylene.
B)para-xylene.
C)phenol.
D)toluene.
E)aniline.

A)meta-xylene.
B)para-xylene.
C)phenol.
D)toluene.
E)aniline.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
The most common reactions involving aromatics are ________ reactions.
A)addition
B)elimination
C)oxidation
D)reduction
E)substitution
A)addition
B)elimination
C)oxidation
D)reduction
E)substitution

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
When the aromatic ring is named as a side chain or functional group,it is referred to as the ________ group.
A)phenyl
B)benzyl
C)xylyl
D)toluyl
E)benzoyl
A)phenyl
B)benzyl
C)xylyl
D)toluyl
E)benzoyl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
Match the following.
resonance
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds
resonance
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Match the following.
polymer
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds
polymer
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Match the following.
phenol
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds
phenol
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
What reagent is used in the bromination of benzene?
A)HNO3,H2SO4
B)FeBr3,Br2
C)HNO3
D)H2SO4
E) H2,Pd
A)HNO3,H2SO4
B)FeBr3,Br2
C)HNO3
D)H2SO4
E) H2,Pd

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
The term delocalization means "not limited to a particular place or area." Explain how this term describes the behavior of electrons in aromatic compounds.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
Match the following.
monomer
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds
monomer
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Match the following.
saturated
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds
saturated
A)another name for 1,2-dichlorobenzene
B)the common name for 1,2-dimethlybenzene
C)another name for 1,3-dichlorobenzene
D)the common name for aminobenzene
E)the common name for hydroxybenzene
F)the term used when a benzene ring is a side chain or substituent group;abbreviated as C6H5-
G)another name for 1,4-dichlorobenzene
H)a term describing a hydrocarbon in which additional C-H bonds can be formed
I)a simple molecule that can be joined with many others to form a large molecule
J)a concept used to describe a molecular structure as an average of two or more similar structures
K)a term describing a hydrocarbon which has the maximum number of C-H bonds possible
L)refers to a class of compounds containing a specific 6-membered ring structure with delocalized electrons
M)a large molecule made from many smaller molecules,often of only one or two kinds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is(are)aromatic compounds?
A)
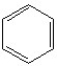
B)
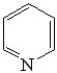
C)
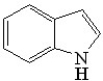
D)None of these are aromatic.
E)All of these are aromatic.
A)

B)

C)

D)None of these are aromatic.
E)All of these are aromatic.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
All of the following are common reactions of benzene except
A)bromination.
B)chlorination.
C)hydrogenation.
D)nitration.
E)sulfonation.
A)bromination.
B)chlorination.
C)hydrogenation.
D)nitration.
E)sulfonation.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
What reagent is used in the sulfonation of benzene?
A)HNO3,H2SO4
B)FeBr3,Br2
C)HNO3
D)H2SO4
E) H2,Pd
A)HNO3,H2SO4
B)FeBr3,Br2
C)HNO3
D)H2SO4
E) H2,Pd

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
What is the name of the following compound? 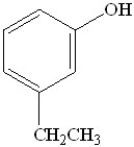
A)o-ethylphenol
B)m-ethylphenol
C)p-ethylphenol
D)m-ethylbenzene

A)o-ethylphenol
B)m-ethylphenol
C)p-ethylphenol
D)m-ethylbenzene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


