Deck 29: Particles and Waves
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/54
Play
Full screen (f)
Deck 29: Particles and Waves
1
An X-ray generator produces photons with energy 49 600 eV or less. Which one of the following phrases most accurately describes the wavelength of these photons?
A)0.025 nm or longer
B)0.050 nm or longer
C)0.75 nm or longer
D)0.25 nm or shorter
E)0.75 nm or shorter
A)0.025 nm or longer
B)0.050 nm or longer
C)0.75 nm or longer
D)0.25 nm or shorter
E)0.75 nm or shorter
0.025 nm or longer
2
Which one of the following statements concerning photons is false?
A)Photons have zero mass.
B)The rest energy of all photons is zero.
C)Photons travel at the speed of light in a vacuum.
D)Photons have been brought to rest by applying a very strong magnetic field to them.
E)The energy of a photon is proportional to its frequency.
A)Photons have zero mass.
B)The rest energy of all photons is zero.
C)Photons travel at the speed of light in a vacuum.
D)Photons have been brought to rest by applying a very strong magnetic field to them.
E)The energy of a photon is proportional to its frequency.
Photons have been brought to rest by applying a very strong magnetic field to them.
3
For which one of the following problems did Max Planck make contributions that eventually led to the development of the "quantum" hypothesis?
A)photoelectric effect
B)uncertainty principle
C)blackbody radiation curves
D)the motion of the earth in the ether
E)the invariance of the speed of light in a vacuum
A)photoelectric effect
B)uncertainty principle
C)blackbody radiation curves
D)the motion of the earth in the ether
E)the invariance of the speed of light in a vacuum
blackbody radiation curves
4
Determine the energy of a single photon in a monochromatic beam of light of wavelength 625 nm.
A)1.99 eV
B)2.08 eV
C)2.32 eV
D)3.49 eV
E)4.77 eV
A)1.99 eV
B)2.08 eV
C)2.32 eV
D)3.49 eV
E)4.77 eV

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
5
Complete the following statement: The photon description of light is necessary to explain
A)polarization
B)photoelectric effect
C)diffraction of light
D)electron diffraction
E)interference of light
A)polarization
B)photoelectric effect
C)diffraction of light
D)electron diffraction
E)interference of light

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
6
Light is usually thought of as wave-like in nature and electrons as particle-like. In which one of the following instances does light behave as a particle or does an electron behave as a wave?
A)A Young's double slit experiment is conducted using blue light.
B)X-rays are used to examine the crystal structure of sodium chloride.
C)Water is heated to 99.0 oC in a microwave oven.
D)An electron enters a parallel plate capacitor, which deflects the electron downward.
E)A beam of electrons is diffracted as it passes through a narrow slit.
A)A Young's double slit experiment is conducted using blue light.
B)X-rays are used to examine the crystal structure of sodium chloride.
C)Water is heated to 99.0 oC in a microwave oven.
D)An electron enters a parallel plate capacitor, which deflects the electron downward.
E)A beam of electrons is diffracted as it passes through a narrow slit.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
7
The graph shows the variation in radiation intensity per unit wavelength versus wavelength for a perfect blackbody at temperature T. 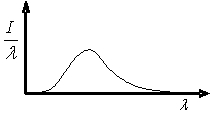 Complete the following statement: As the blackbody temperature is increased, the peak in intensity of this curve
Complete the following statement: As the blackbody temperature is increased, the peak in intensity of this curve
A)will remain constant.
B)will be shifted to longer wavelengths and its magnitude will increase.
C)will be shifted to shorter wavelengths and its magnitude will increase.
D)will be shifted to longer wavelengths and its magnitude will decrease.
E)will be shifted to shorter wavelengths and its magnitude will decrease.
 Complete the following statement: As the blackbody temperature is increased, the peak in intensity of this curve
Complete the following statement: As the blackbody temperature is increased, the peak in intensity of this curveA)will remain constant.
B)will be shifted to longer wavelengths and its magnitude will increase.
C)will be shifted to shorter wavelengths and its magnitude will increase.
D)will be shifted to longer wavelengths and its magnitude will decrease.
E)will be shifted to shorter wavelengths and its magnitude will decrease.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following phrases best describes the term work function?
A)the minimum energy required to vaporize a metal surface
B)the work required to place a charged particle on a metal surface
C)the minimum energy required to remove electrons from a metal surface
D)the minimum energy required to remove an atom from a metal surface
E)the work done by electromagnetic radiation when it hits a metal surface
A)the minimum energy required to vaporize a metal surface
B)the work required to place a charged particle on a metal surface
C)the minimum energy required to remove electrons from a metal surface
D)the minimum energy required to remove an atom from a metal surface
E)the work done by electromagnetic radiation when it hits a metal surface

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
9
Electrons are emitted from a certain metal with a maximum kinetic energy of 2 eV when 6-eV photons are incident on its surface. If photons of twice the wavelength are incident on this metal which one of the following statements is true?
A)No electrons will be emitted.
B)Electrons will be emitted with a maximum kinetic energy of 3 eV.
C)Electrons will be emitted with a maximum kinetic energy of 6 eV.
D)Electrons will be emitted with a maximum kinetic energy of 9 eV.
E)Electrons will be emitted with a maximum kinetic energy of 12 eV.
A)No electrons will be emitted.
B)Electrons will be emitted with a maximum kinetic energy of 3 eV.
C)Electrons will be emitted with a maximum kinetic energy of 6 eV.
D)Electrons will be emitted with a maximum kinetic energy of 9 eV.
E)Electrons will be emitted with a maximum kinetic energy of 12 eV.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
10
Upon which one of the following parameters does the energy of a photon depend?
A)mass
B)amplitude
C)polarization
D)frequency
E)phase relationships
A)mass
B)amplitude
C)polarization
D)frequency
E)phase relationships

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
11
A laser emits a single, 3.0-ms pulse of light that has a frequency of 2.83 × 1011 Hz and a total power of 65 000 W. How many photons are in the pulse?
A)6.0 × 1023
B)1.2 × 1024
C)2.4 × 1025
D)3.6 × 1025
E)4.8 × 1026
A)6.0 × 1023
B)1.2 × 1024
C)2.4 × 1025
D)3.6 × 1025
E)4.8 × 1026

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
12
Complete the following statement: The term photon applies
A)only to X-rays.
B)only to visible light.
C)to any form of wave motion.
D)to any form of particle motion.
E)to any form of electromagnetic radiation.
A)only to X-rays.
B)only to visible light.
C)to any form of wave motion.
D)to any form of particle motion.
E)to any form of electromagnetic radiation.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
13
Photons of what minimum frequency are required to remove electrons from gold? Note: The work function for gold is 4.8 eV.
A)7.3 × 1014 Hz
B)1.2 × 1015 Hz
C)3.8 × 1017 Hz
D)6.5 × 1015 Hz
E)4.6 × 1014 Hz
A)7.3 × 1014 Hz
B)1.2 × 1015 Hz
C)3.8 × 1017 Hz
D)6.5 × 1015 Hz
E)4.6 × 1014 Hz

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
14
A laser emits a 5.0 × 103-J pulse of light that has a wavelength of 480 nm. Determine the number of photons in the pulse.
A)5.2 × 1016
B)2.5 × 1019
C)1.2 × 1022
D)3.1 × 1022
E)8.1 × 1024
A)5.2 × 1016
B)2.5 × 1019
C)1.2 × 1022
D)3.1 × 1022
E)8.1 × 1024

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
15
A laser produces 3.0 W of light that has a wavelength of 600 nm. How many photons per second are produced?
A)7.3 × 1015
B)4.2 × 1017
C)1.0 × 1017
D)3.0 × 1018
E)9.1 × 1018
A)7.3 × 1015
B)4.2 × 1017
C)1.0 × 1017
D)3.0 × 1018
E)9.1 × 1018

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
16
White light consisting of wavelengths 380 nm 750 nm is incident on a lead surface. For which one of the following ranges of wavelengths will photoelectrons be emitted from the lead surface that has a work function W0 = 6.63 × 10-19 J?
A)380 nm 750 nm
B)380 nm 630 nm
C)380 nm 540 nm
D)380 nm 410 nm
E)No photoelectrons will be emitted.
A)380 nm 750 nm
B)380 nm 630 nm
C)380 nm 540 nm
D)380 nm 410 nm
E)No photoelectrons will be emitted.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following quantities is the same for all photons in vacuum?
A)speed
B)frequency
C)kinetic energy
D)wavelength
E)total energy
A)speed
B)frequency
C)kinetic energy
D)wavelength
E)total energy

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
18
Which type of wave motion does not involve photons?
A)gamma rays
B)microwaves
C)radio waves
D)infrared radiation
E)sound waves
A)gamma rays
B)microwaves
C)radio waves
D)infrared radiation
E)sound waves

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
19
A laser emits photons of energy 5.0 eV with a power of 10-2 W. How many photons are emitted each second?
A)4.0 × 1014
B)2.7 × 1015
C)5.0 × 1018
D)3.1 × 1021
E)2.5 × 1021
A)4.0 × 1014
B)2.7 × 1015
C)5.0 × 1018
D)3.1 × 1021
E)2.5 × 1021

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
20
When ultraviolet photons with a wavelength of 3.45 × 10-7 m are directed at the surface of an unknown metal in vacuum, electrons with a maximum kinetic energy of 1.52 eV are emitted from the surface. What is the work function of the metal?
A)3.60 eV
B)3.11 eV
C)2.59 eV
D)2.08 eV
E)1.98 eV
A)3.60 eV
B)3.11 eV
C)2.59 eV
D)2.08 eV
E)1.98 eV

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
21
A physicist wishes to produce electrons by shining light on a metal surface. The light source emits light with a wavelength of 450 nm. The table lists the only available metals and their work functions. 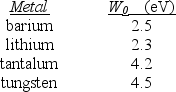
Which entry in the table below correctly identifies the metal that will produce the most energetic electrons and their energies?
A)
B)
C)
D)
E)

Which entry in the table below correctly identifies the metal that will produce the most energetic electrons and their energies?
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
22
Incident X-rays in a Compton scattering experiment have a wavelength of 0.400 nm. Determine the wavelength of the scattered X-rays that are detected at an angle of 80.0°. The Compton wavelength of the electron is 2.43 × 10-12 m.
A)0.041 nm
B)0.398 nm
C)0.399 nm
D)0.402 nm
E)0.403 nm
A)0.041 nm
B)0.398 nm
C)0.399 nm
D)0.402 nm
E)0.403 nm

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
23
The work function for a particular metal is 4.0 eV. Which one of the following best describes the wavelength of electromagnetic radiation needed to eject electrons from this metal?
A)310 nm or greater
B)310 nm or smaller
C)620 nm or greater
D)620 nm or smaller
E)800 nm or greater
A)310 nm or greater
B)310 nm or smaller
C)620 nm or greater
D)620 nm or smaller
E)800 nm or greater

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
24
Complete the following statement: According to the de Broglie relation, the wavelength of a "matter" wave is inversely proportional to
A)Planck's constant.
B)the mass of the particle.
C)the momentum of the particle.
D)the frequency of the wave.
E)the speed of the particle.
A)Planck's constant.
B)the mass of the particle.
C)the momentum of the particle.
D)the frequency of the wave.
E)the speed of the particle.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
25
In the Compton effect, a photon of wavelength and frequency f hits an electron that is initially at rest. Which one of the following occurs as a result of the collision?
A)The photon is absorbed completely.
B)The photon gains energy, so the final photon has a frequency greater than f.
C)The photon gains energy, so the final photon has a wavelength greater than .
D)The photon loses energy, so the final photon has a frequency less than f.
E)The photon loses energy, so the final photon has a wavelength less than .
A)The photon is absorbed completely.
B)The photon gains energy, so the final photon has a frequency greater than f.
C)The photon gains energy, so the final photon has a wavelength greater than .
D)The photon loses energy, so the final photon has a frequency less than f.
E)The photon loses energy, so the final photon has a wavelength less than .

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
26
A physicist wishes to produce electrons by shining light on a metal surface. The light source emits light with a wavelength of 450 nm. The table lists the only available metals and their work functions. 
Which metal(s) can be used to produce electrons by the photoelectric effect?
A)barium only
B)tungsten only
C)tungsten or tantalum
D)barium or lithium
E)lithium, tantalum, or tungsten

Which metal(s) can be used to produce electrons by the photoelectric effect?
A)barium only
B)tungsten only
C)tungsten or tantalum
D)barium or lithium
E)lithium, tantalum, or tungsten

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
27
Photons of energy 5.0 eV strike a metal surface that has a work function of 3.5 eV. Determine which one of the following best describes the kinetic energy of the emitted electrons.
A)1.5 eV or less
B)1.5 eV or more, but less than 2.5 eV
C)2.5 eV or more, but less than 3.5 eV
D)3.5 eV or more
E)3.5 eV or less, but more than 1.5 eV
A)1.5 eV or less
B)1.5 eV or more, but less than 2.5 eV
C)2.5 eV or more, but less than 3.5 eV
D)3.5 eV or more
E)3.5 eV or less, but more than 1.5 eV

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
28
Complete the following statement: The photon or "particle" theory of electromagnetic radiation is necessary to explain the
A)refraction of light by a prism.
B)diffraction of light by a grating.
C)reflection of light from a mirrored surface.
D)results of Compton scattering experiments.
E)interference of light in Young's double-slit experiment.
A)refraction of light by a prism.
B)diffraction of light by a grating.
C)reflection of light from a mirrored surface.
D)results of Compton scattering experiments.
E)interference of light in Young's double-slit experiment.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
29
Which experimental evidence confirms the hypothesis that matter exhibits wave properties?
A)photoelectric experiments
B)electron diffraction experiments
C)Young's double-slit experiments
D)Compton scattering experiments
E)Michelson-Morley experiment
A)photoelectric experiments
B)electron diffraction experiments
C)Young's double-slit experiments
D)Compton scattering experiments
E)Michelson-Morley experiment

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
30
What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) in a 2.5 × 103-volt X-ray tube?
A)0.0072 nm
B)0.011 nm
C)0.017 nm
D)0.031 nm
E)0.025 nm
A)0.0072 nm
B)0.011 nm
C)0.017 nm
D)0.031 nm
E)0.025 nm

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
31
Determine the de Broglie wavelength of a neutron (m = 1.67 × 10-27 kg) that has a speed of 5.0 m/s.
A)79 nm
B)162 nm
C)395 nm
D)529 nm
E)1980 nm
A)79 nm
B)162 nm
C)395 nm
D)529 nm
E)1980 nm

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
32
A photon has a collision with a stationary electron (h/mc = 2.43 × 10-12 m) and loses 5.0% of its energy. The photon scattering angle is 180°. What is the wavelength of the incident photon in this scattering process?
A)2.4 × 10-12 m
B)4.6 × 10-11 m
C)9.2 × 10-11 m
D)1.9 × 10-10 m
E)3.1 × 10-12 m
A)2.4 × 10-12 m
B)4.6 × 10-11 m
C)9.2 × 10-11 m
D)1.9 × 10-10 m
E)3.1 × 10-12 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
33
What is the speed of an electron that has the same momentum as a photon with a wavelength in vacuum of 488 nm? The mass of an electron is 9.11 × 10-31 kg.
A)1.5 × 103 m/s
B)1.9 × 103 m/s
C)2.1 × 103 m/s
D)2.3 × 103 m/s
E)2.9 × 103 m/s
A)1.5 × 103 m/s
B)1.9 × 103 m/s
C)2.1 × 103 m/s
D)2.3 × 103 m/s
E)2.9 × 103 m/s

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
34
The de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) is 1.2 × 10-10 m. Determine the kinetic energy of the electron.
A)1.5 × 10-15 J
B)1.6 × 10-16 J
C)1.7 × 10-17 J
D)1.8 × 10-18 J
E)1.9 × 10-19 J
A)1.5 × 10-15 J
B)1.6 × 10-16 J
C)1.7 × 10-17 J
D)1.8 × 10-18 J
E)1.9 × 10-19 J

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
35
A photon of wavelength 200 nm is scattered by an electron that is initially at rest. Which one of the following statements concerning the wavelength of the scattered photon is true?
A)The wavelength is zero nm.
B)The wavelength is 200 nm.
C)The wavelength is 100 nm.
D)The wavelength is greater than 200 nm.
E)The wavelength is less than 200 nm, but greater than zero.
A)The wavelength is zero nm.
B)The wavelength is 200 nm.
C)The wavelength is 100 nm.
D)The wavelength is greater than 200 nm.
E)The wavelength is less than 200 nm, but greater than zero.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
36
A digital wireless telephone communicates to a base via microwaves with a frequency of 1930 MHz. What are the momentum and energy for the microwave photons emitted by the telephone? 
A)
B)
C)
D)
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
37
In the Compton scattering experiment shown in the figure, a monochromatic beam of X-rays strikes a target containing free electrons. Scattered X-rays are detected with a wavelength of 2.50 × 10-12 m at an angle of 45° away from the original beam direction. What is the wavelength of the incident monochromatic X-rays? Note: The mass of an electron is 9.11 × 10-31 kg. 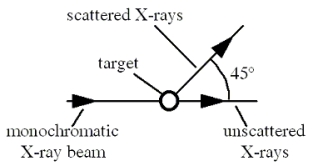
A)3.21 × 10-12 m
B)2.86 × 10-12 m
C)2.50 × 10-12 m
D)2.03 × 10-12 m
E)1.79 × 10-12 m

A)3.21 × 10-12 m
B)2.86 × 10-12 m
C)2.50 × 10-12 m
D)2.03 × 10-12 m
E)1.79 × 10-12 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is demonstrated by the Compton effect?
A)time dilation
B)length contraction
C)the uncertainty principle
D)the wave properties of electrons
E)the particle properties of electromagnetic radiation
A)time dilation
B)length contraction
C)the uncertainty principle
D)the wave properties of electrons
E)the particle properties of electromagnetic radiation

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
39
Approximately, what is the de Broglie wavelength of an electron that has been accelerated through a potential difference of 225 V? The mass of an electron is 9.11 × 10-31 kg.
A)0.14 nm
B)0.082 nm
C)0.043 nm
D)0.0092 nm
E)0.22 nm
A)0.14 nm
B)0.082 nm
C)0.043 nm
D)0.0092 nm
E)0.22 nm

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
40
What kinetic energy must each neutron in a beam of neutrons have if each has a wavelength of 0.10 nm? The mass of a neutron is 1.67 × 10-27 kg.
A)6.6 × 10-19 J
B)1.3 × 10-20 J
C)2.6 × 10-20 J
D)6.3 × 10-20 J
E)7.1 × 10-20 J
A)6.6 × 10-19 J
B)1.3 × 10-20 J
C)2.6 × 10-20 J
D)6.3 × 10-20 J
E)7.1 × 10-20 J

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
41
The speed of a bullet with a mass of 0.050 kg is 420 m/s with an uncertainty of 0.010 %. What is the minimum uncertainty in the bullet's position if it is measured at the same time as the speed is measured?
A)2.5 × 10-31 m
B)5.0 × 10-32 m
C)7.5 × 10-33 m
D)2.5 × 10-34 m
E)6.0 × 10-36 m
A)2.5 × 10-31 m
B)5.0 × 10-32 m
C)7.5 × 10-33 m
D)2.5 × 10-34 m
E)6.0 × 10-36 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
42
It is desired to obtain a diffraction pattern for electrons using a diffraction grating with lines separated by 10 nm. The mass of an electron is 9.11 × 10-31 kg.
What is the approximate kinetic energy of electrons that would be diffracted by such a grating?
A)1.5 × 10-6 eV
B)1.5 × 10-4 eV
C)1.5 × 10-2 eV
D)1.5 × 102 eV
E)1.5 × 108 eV
What is the approximate kinetic energy of electrons that would be diffracted by such a grating?
A)1.5 × 10-6 eV
B)1.5 × 10-4 eV
C)1.5 × 10-2 eV
D)1.5 × 102 eV
E)1.5 × 108 eV

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
43
The position of a hydrogen atom (m = 1.7 × 10-27 kg) is known to within 2.0 × 10-6 m. What is the minimum uncertainty in the atom's velocity?
A)zero m/s
B)0.0085 m/s
C)0.011 m/s
D)0.016 m/s
E)0.031 m/s
A)zero m/s
B)0.0085 m/s
C)0.011 m/s
D)0.016 m/s
E)0.031 m/s

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
44
A proton (mp = 1.673 × 10-27 kg) and an electron (me = 9.109 × 10-31 kg) are confined such that the x position of each is known to within 1.50 × 10-10 m. What is the ratio of the minimum uncertainty in the x component of the velocity of the electron to that of the proton, ve/ vp?
A)5.444 × 10-4
B)0.2272
C)808.2
D)1837
E)36 290
A)5.444 × 10-4
B)0.2272
C)808.2
D)1837
E)36 290

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
45
What is the kinetic energy of each electron in a beam of electrons if the beam produces a diffraction pattern of a crystal which is similar to that of a beam of 1.00 eV neutrons? Note: The electron mass is 9.11 × 10-31 kg; and the neutron mass is 1.67 × 10-27 kg.
A)1830 eV
B)42.8 eV
C)3.35 × 106 eV
D)5.46 × 10-4 eV
E)2.34 × 10-2 eV
A)1830 eV
B)42.8 eV
C)3.35 × 106 eV
D)5.46 × 10-4 eV
E)2.34 × 10-2 eV

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
46
In a computer monitor, electrons approach the screen at 1.20 × 108 m/s. What is the de Broglie wavelength of these electrons? Note: the mass of electrons is 9.109 × 10-31 kg; and use the relativistic momentum in your calculation.
A)4.31 × 10-12 m
B)5.56 × 10-12 m
C)6.07 × 10-12 m
D)6.62 × 10-12 m
E)7.85 × 10-12 m
A)4.31 × 10-12 m
B)5.56 × 10-12 m
C)6.07 × 10-12 m
D)6.62 × 10-12 m
E)7.85 × 10-12 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
47
In an experiment to determine the speed and position of an electron (m = 9.11 × 10-31 kg), three researchers claim to have measured the position of the electron to within ± 10-9 m. They reported the following values for the speed of the electron: 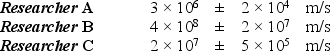 Which of these measurements violates one or more basic laws of modern physics?
Which of these measurements violates one or more basic laws of modern physics?
A)A only
B)B only
C)A and B
D)B and C
E)A, B, and C
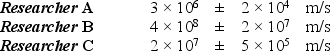 Which of these measurements violates one or more basic laws of modern physics?
Which of these measurements violates one or more basic laws of modern physics?A)A only
B)B only
C)A and B
D)B and C
E)A, B, and C

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
48
The position of a 1-g object moving in the x direction at 1 cm/s is known to within ± 10 nm. In which range is the fractional uncertainty, ( px)/px , in the x component of its momentum?
A)10-2 to 10-4
B)10-12 to 10-14
C)10-16 to 10-18
D)10-20 to 10-22
E)less than 10-30
A)10-2 to 10-4
B)10-12 to 10-14
C)10-16 to 10-18
D)10-20 to 10-22
E)less than 10-30

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
49
What happens to the de Broglie wavelength of an electron if its momentum is reduced to one-half of its initial value?
A)The wavelength decreases by a factor of 4.
B)The wavelength increases by a factor of 4.
C)The wavelength increases by a factor of 3.
D)The wavelength increases by a factor of 2.
E)The wavelength decreases by a factor of 2.
A)The wavelength decreases by a factor of 4.
B)The wavelength increases by a factor of 4.
C)The wavelength increases by a factor of 3.
D)The wavelength increases by a factor of 2.
E)The wavelength decreases by a factor of 2.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
50
A beam of electrons is incident on a single slit that has a width of 1.00 × 10-6 m and a diffraction pattern is observed on a screen located 15.0 m from the slit. The momentum of an individual electron in the beam is 5.91 × 10-24 kg . m/s. What is the width of the central maximum fringe?
A)5.87 × 10-5 m
B)1.33 × 10-4 m
C)6.67 × 10-4 m
D)3.37 × 10-3 m
E)7.50 × 10-3 m
A)5.87 × 10-5 m
B)1.33 × 10-4 m
C)6.67 × 10-4 m
D)3.37 × 10-3 m
E)7.50 × 10-3 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
51
The x component of the velocity of an electron (m = 9.11 × 10-31 kg) is known to be between 100 m/s and 300 m/s. Which one of the following is a true statement concerning the uncertainty in the x coordinate of the electron?
A)The maximum uncertainty is about 106 m.
B)The minimum uncertainty is about 3 × 10-7 m.
C)The maximum uncertainty is about 6 × 10-7 m.
D)The minimum uncertainty is about 3 × 10-9 m.
E)The maximum uncertainty is about 6 × 10-9 m.
A)The maximum uncertainty is about 106 m.
B)The minimum uncertainty is about 3 × 10-7 m.
C)The maximum uncertainty is about 6 × 10-7 m.
D)The minimum uncertainty is about 3 × 10-9 m.
E)The maximum uncertainty is about 6 × 10-9 m.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
52
If Planck's constant were changed to 660 J . s, what would be the minimum uncertainty in the position of a 120-kg football player running at a speed of 3.5 m/s?
A)0.032 m
B)0.065 m
C)0.13 m
D)0.25 m
E)0.50 m
A)0.032 m
B)0.065 m
C)0.13 m
D)0.25 m
E)0.50 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
53
The Hubble Space Telescope has an orbital speed of 7.56 × 103 m/s and a mass of 11 600 kg. What is the de Broglie wavelength of the telescope?
A)8.77 × 107 m
B)5.81 × 10-26 m
C)6.63 × 10-34 m
D)3.78 × 10-40 m
E)7.56 × 10-42 m
A)8.77 × 107 m
B)5.81 × 10-26 m
C)6.63 × 10-34 m
D)3.78 × 10-40 m
E)7.56 × 10-42 m

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
54
It is desired to obtain a diffraction pattern for electrons using a diffraction grating with lines separated by 10 nm. The mass of an electron is 9.11 × 10-31 kg.
Suppose it is desired to observe diffraction effects for a beam of electromagnetic radiation using the same grating. Roughly, what is the required energy of the individual photons in the beam?
A)10-6 eV
B)10-4 eV
C)10-2 eV
D)102 eV
E)104 eV
Suppose it is desired to observe diffraction effects for a beam of electromagnetic radiation using the same grating. Roughly, what is the required energy of the individual photons in the beam?
A)10-6 eV
B)10-4 eV
C)10-2 eV
D)102 eV
E)104 eV

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck


