Deck 5: The Properties of Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 5: The Properties of Gases
1
A pressure of 2.50 105 kgm1s2 corresponds to
A)1.00 atm
B)760 mmHg
C)253 kPa
D)2.50 105 Torr
E)2.50 Pa
A)1.00 atm
B)760 mmHg
C)253 kPa
D)2.50 105 Torr
E)2.50 Pa
253 kPa
2
The height of water in a water barometer is 883 cm at 20C.The density of water at 20C is 0.998 gcm3.What is the pressure?
A)88.3 kPa
B)8.81 103 Pa
C)101 kPa
D)8.64 103 Pa
E)86.4 kPa
A)88.3 kPa
B)8.81 103 Pa
C)101 kPa
D)8.64 103 Pa
E)86.4 kPa
86.4 kPa
3
What is the density of helium gas at 750 kPa and 25C?
A)2.42 gL1
B)1.21 gL1
C)0.605 gL1
D)0.161 gL1
E)0.0120 gL1
A)2.42 gL1
B)1.21 gL1
C)0.605 gL1
D)0.161 gL1
E)0.0120 gL1
1.21 gL1
4
All of the following elements are gases at room temperature and atmospheric pressure except
A)Rn
B)F
C)Br
D)N
E)Cl
A)Rn
B)F
C)Br
D)N
E)Cl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
What is the pressure inside a system when the system-side column in an open mercury manometer is 75.0 mm lower than the atmosphere side when the atmospheric pressure is 735 mmHg?
A)820 kPa
B)10.0 kPa
C)88.0 kPa
D)75 kPa
E)108 kPa
A)820 kPa
B)10.0 kPa
C)88.0 kPa
D)75 kPa
E)108 kPa

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
The height of mercury in a barometer is 77.5 cm.What height would water reach in a water barometer? The density of mercury is 13.6 gcm3 and that of water 1.00 gcm3.The temperature is constant.
A)175 cm
B)55.9 cm
C)760 cm
D)5.70 cm
E)1.05 103 cm
A)175 cm
B)55.9 cm
C)760 cm
D)5.70 cm
E)1.05 103 cm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
A badly tuned automobile engine can release about 50 moles of carbon dioxide per hour.At 35C,what volume of carbon dioxide is released in a six-hour period if the atmospheric pressure is 740 Torr?
A)7.9 105 L
B)7.8 103 L
C)1.3 103 L
D)10 L
E)1.3 105 L
A)7.9 105 L
B)7.8 103 L
C)1.3 103 L
D)10 L
E)1.3 105 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
What is the density of xenon gas at 750 kPa and 25C?
A)5.30 gL1
B)0.392 gL1
C)39.7 gL1
D)79.5 gL1
E)19.9 gL1
A)5.30 gL1
B)0.392 gL1
C)39.7 gL1
D)79.5 gL1
E)19.9 gL1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
The number of molecules in 44.8 L of nitrogen at exactly 0C and 3.00 atm is
A)1.20 1024
B)3.61 1024
C)3.61 10-23
D)2.01 1023
E)18.0 1023
A)1.20 1024
B)3.61 1024
C)3.61 10-23
D)2.01 1023
E)18.0 1023

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
If 2.00 moles of an ideal gas at STP is subjected to a new pressure of 24.7 kPa,the volume of the gas will become
A)8.20 L
B)22.4 L
C)11.2 L
D)184 L
E)91.9 L
A)8.20 L
B)22.4 L
C)11.2 L
D)184 L
E)91.9 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
A sample of gas with a volume of 750 mL exerts a pressure of 98.0 kPa at 30C.What pressure will the sample exert when it is compressed to 250 mL and cooled to 25C?
A)353 kPa
B)241 kPa
C)359 kPa
D)39.9 kPa
E)26.7 kPa
A)353 kPa
B)241 kPa
C)359 kPa
D)39.9 kPa
E)26.7 kPa

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
An oxygen tank kept at 20C contains 28.0 moles of oxygen and the gauge reads 31.0 atm.After two weeks,the gauge reads 10.5 atm.How many moles of oxygen were used during the two-week period?
A)9.25 moles
B)7.5 moles
C)20.5 moles
D)18.5 moles
E)9.48 moles
A)9.25 moles
B)7.5 moles
C)20.5 moles
D)18.5 moles
E)9.48 moles

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
How many atoms of argon occupy 20.00 mL if the temperature is 200°C and the pressure is 2.00 × 10-3 Torr?
A)2.01 1017
B)6.02 10-17
C)3.10 1017
D)2.44 1019
E)2.80 1011
A)2.01 1017
B)6.02 10-17
C)3.10 1017
D)2.44 1019
E)2.80 1011

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
The pressure at 20,000 feet above sea level is about 400.mmHg.This corresponds to
A)526 Pa
B)53.3 kgm1s2
C)0.526 Torr
D)0.526 kPa
E)53.3 kPa
A)526 Pa
B)53.3 kgm1s2
C)0.526 Torr
D)0.526 kPa
E)53.3 kPa

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the pressure of 4.00 g of nitrogen gas in a 1.00-L container at 20.0C.
A)0.469 atm
B)6.87 atm
C)96.2 atm
D)0.235 atm
E)3.44 atm
A)0.469 atm
B)6.87 atm
C)96.2 atm
D)0.235 atm
E)3.44 atm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
The value of the gas law constant,R,in units of LkPamol1K1 is
A)62.4
B)0.0821
C)0.0752
D)7.62
E)8.31
A)62.4
B)0.0821
C)0.0752
D)7.62
E)8.31

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
The value of the gas law constant,R,in units of m3Pamol1K1 is
A)8.31
B)0.0821
C)7.62
D)0.0752
E)62.4
A)8.31
B)0.0821
C)7.62
D)0.0752
E)62.4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
What volume is occupied by 1,000.g of fluorine gas at 10.00°C at a pressure of 735 Torr?
A)22.3 L
B)44.6 L
C)621 L
D)632 L
E)4,690 L
A)22.3 L
B)44.6 L
C)621 L
D)632 L
E)4,690 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
What volume is occupied by 1.00 kg of helium at 5.00C at a pressure of 735 Torr?
A)5.97 105 L
B)5.90 103 L
C)2.95 103 L
D)1.06 102 L
E)5.60 103 L
A)5.97 105 L
B)5.90 103 L
C)2.95 103 L
D)1.06 102 L
E)5.60 103 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the mass of oxygen gas required to occupy a volume of 6.00 L at a pressure of 20.9 kPa and a temperature of 37.0C.
A)1.56 g
B)0.408 g
C)0.779 g
D)13.1 g
E)0.0487 g
A)1.56 g
B)0.408 g
C)0.779 g
D)13.1 g
E)0.0487 g

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following reaction: 4KO2(s)+ 2CO2(g) 2K2CO3(s)+ 3O2(g)
How many liters of oxygen are produced at STP if 10.5 moles of carbon dioxide are used at STP?
A)15.8
B)0.703
C)706
D)353
E)235
How many liters of oxygen are produced at STP if 10.5 moles of carbon dioxide are used at STP?
A)15.8
B)0.703
C)706
D)353
E)235

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
A 0.479-g sample of nitrogen,oxygen or neon gas occupies a volume of 265 mL at 157 l Pa and 20.0C.What are the molar mass and identity of the gas?
A)14.0 gmol1, N
B)32.0 gmol1, O2
C)20.1 gmol1, Ne
D)16.0 gmol1, O
E)28.0 gmol1, N2
A)14.0 gmol1, N
B)32.0 gmol1, O2
C)20.1 gmol1, Ne
D)16.0 gmol1, O
E)28.0 gmol1, N2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
The density of citronellal,a mosquito repellant,is 1.45 gL1 at 365C and 50.0 kPa.What is the molar mass of citronellal?
A)73.2 gmol1
B)154 gmol1
C)95.7 gmol1
D)37.5 gmol1
E)88.0 gmol1
A)73.2 gmol1
B)154 gmol1
C)95.7 gmol1
D)37.5 gmol1
E)88.0 gmol1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the number of moles of oxygen gas collected by displacement of water at 14.0C if the atmospheric pressure is 790.Torr and the volume is 5.00 L.The vapor pressure of water at 14.0C is 12.0 Torr.
A)0.0184
B)4.46
C)0.00335
D)0.217
E)0.224
A)0.0184
B)4.46
C)0.00335
D)0.217
E)0.224

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
If 250.0 mL of a gas at STP weighs 2.00 g,what is the molar mass of the gas?
A)28.0 gmol1
B)179 gmol1
C)8.00 gmol1
D)56.0 gmol1
E)44.8 gmol1
A)28.0 gmol1
B)179 gmol1
C)8.00 gmol1
D)56.0 gmol1
E)44.8 gmol1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
What is the molar mass of a gas whose density is 3.55 gL1 at 110C and a pressure of 736 Torr?
A)15.4 gmol1
B)79.5 gmol1
C)115 gmol1
D)152 gmol1
E)33.1 gmol1
A)15.4 gmol1
B)79.5 gmol1
C)115 gmol1
D)152 gmol1
E)33.1 gmol1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Which gas is least dense at 1 atm and 25C?
A)Hydrogen cyanide
B)Hydrogen sulfide
C)Carbon dioxide
D)Nitrogen
E)Ammonia
A)Hydrogen cyanide
B)Hydrogen sulfide
C)Carbon dioxide
D)Nitrogen
E)Ammonia

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the density of camphor,C10H16O,at 80C and 12 Torr.
A)8.2 104 gL1
B)0.083 gL1
C)0.37 gL1
D)0.62 gL1
E)6.8 10-3 gL1
A)8.2 104 gL1
B)0.083 gL1
C)0.37 gL1
D)0.62 gL1
E)6.8 10-3 gL1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Ammonium nitrate can decompose according to the following equation: NH4NO3(s) N2O(g)+ 2H2O(g)
How many liters of gas are produced by decomposition of 160 g of ammonium nitrate at STP?
A)44.8
B)6.00
C)22.4
D)134
E)67.2
How many liters of gas are produced by decomposition of 160 g of ammonium nitrate at STP?
A)44.8
B)6.00
C)22.4
D)134
E)67.2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
What is the density of chlorine gas at 850 Torr and 12C?
A)27.8 gL1
B)3.70 gL1
C)3.39 gL1
D)7.40 gL1
E)1.85 gL1
A)27.8 gL1
B)3.70 gL1
C)3.39 gL1
D)7.40 gL1
E)1.85 gL1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
A sample of a gas weighing 15.1 g occupies 2.25 L at 1.75 atm and 20.0C.If the empirical formula of the gas is NO2,what is the molecular formula?
A)N5O10
B)N3O6
C)NO2
D)N4O8
E)N2O4
A)N5O10
B)N3O6
C)NO2
D)N4O8
E)N2O4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
Which gas is most dense at 1 atm and 25C?
A)Hydrogen cyanide
B)Hydrogen sulfide
C)Nitrogen monoxide
D)Carbon monoxide
E)Nitrogen dioxide
A)Hydrogen cyanide
B)Hydrogen sulfide
C)Nitrogen monoxide
D)Carbon monoxide
E)Nitrogen dioxide

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
At 80.0C and 12.0 Torr,the density of camphor vapor is 0.0829 gL1.What is the molar mass of camphor?
A)243 gmol1
B)34.5 gmol1
C)152 gmol1
D)3490 gmol1
E)20.3 gmol1
A)243 gmol1
B)34.5 gmol1
C)152 gmol1
D)3490 gmol1
E)20.3 gmol1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Ammonium nitrate can decompose according to the following equation: NH4NO3(s) N2O(g)+ 2H2O(g)
How many liters of gas are produced by decomposition of 160 g of ammonium nitrate at 25C and 86.0 kPa?
A)48.0
B)14.5
C)57.6
D)173
E)144
How many liters of gas are produced by decomposition of 160 g of ammonium nitrate at 25C and 86.0 kPa?
A)48.0
B)14.5
C)57.6
D)173
E)144

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following reaction: 4KO2(s)+ 2CO2(g) 2K2CO3(s)+ 3O2(g)
How many moles of KO2 are needed to react with 75.0 L of carbon dioxide at 25C and 215 kPa?
A)3.91
B)7.82
C)31.3
D)23.5
E)15.6
How many moles of KO2 are needed to react with 75.0 L of carbon dioxide at 25C and 215 kPa?
A)3.91
B)7.82
C)31.3
D)23.5
E)15.6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
What is the density of nitrogen gas at 740 Torr and 12C?
A)0.637 gL1
B)1.17 gL1
C)1.27 gL1
D)2.55 gL1
E)9.56 gL1
A)0.637 gL1
B)1.17 gL1
C)1.27 gL1
D)2.55 gL1
E)9.56 gL1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following reaction: 4NaO2(s)+ 2CO2(g) 2Na2CO3(s)+ 3O2(g)
How many moles of NaO2 are needed to react with 75.0 L of carbon dioxide at STP?
A)0.15
B)1.67
C)3.35
D)6.70
E)13.4
How many moles of NaO2 are needed to react with 75.0 L of carbon dioxide at STP?
A)0.15
B)1.67
C)3.35
D)6.70
E)13.4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
A Group 17 or Group 18 gas has a density of 2.92 gL1 at 1.00 atm and 25C.The gas is
A)helium
B)neon
C)fluorine
D)argon
E)chlorine
A)helium
B)neon
C)fluorine
D)argon
E)chlorine

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
The density of the vapor of allicin,a component of garlic,is 1.14 gL1 at 125C and 175 Torr.What is the molar mass of allicin?
A)21.6 gmol1
B)50.8 gmol1
C)273 gmol1
D)869 gmol1
E)162 gmol1
A)21.6 gmol1
B)50.8 gmol1
C)273 gmol1
D)869 gmol1
E)162 gmol1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
The empirical formula of a gas is CH3O.If 2.77 g of the gas occupies 1.00 L at exactly 0C at a pressure of 760 Torr,what is the molecular formula of the gas?
A)C4H12O4
B)C2H6O2
C)C5H15O5
D)CH3O
E)C3H9O3
A)C4H12O4
B)C2H6O2
C)C5H15O5
D)CH3O
E)C3H9O3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Calcium cyanamide reacts with H2O(g)at 150C and 1.00 atm pressure: CaNCN(s)+ 3H2O(g) CaCO3(s)+ 2NH3(g)
If 80.0 L of NH3(g)are produced,what volume of steam is required?
A)40.0 L
B)80.0 L
C)26.7 L
D)120. L
E)240. L
If 80.0 L of NH3(g)are produced,what volume of steam is required?
A)40.0 L
B)80.0 L
C)26.7 L
D)120. L
E)240. L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
The following experiment was carried out using a newly synthesized chlorofluorocarbon.Exactly 50 mL of the gas effused through a porous barrier in 157 s.The same volume of argon effused in 76 s under the same conditions.Which compound is the chlorofluorocarbon?
A)C2Cl4F2
B)C2ClF5
C)C2Cl2F4
D)C2Cl5F
E)C2Cl3F3
A)C2Cl4F2
B)C2ClF5
C)C2Cl2F4
D)C2Cl5F
E)C2Cl3F3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
Sulfur dioxide reacts with oxygen gas to produce sulfur trioxide.How many moles of oxygen gas will react with 15.0 L of sulfur dioxide if both gases are at 101.3 kPa and 125C?
A)0.230
B)0.115
C)0.731
D)0.919
E)0.459
A)0.230
B)0.115
C)0.731
D)0.919
E)0.459

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
A sample of nitrogen gas collected at 24C and 745 Torr has a vapor pressure of 745 Torr.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following gases effuses slowest?
A)Fluorine
B)Carbon dioxide
C)Chlorine
D)Carbon monoxide
E)Nitrogen
A)Fluorine
B)Carbon dioxide
C)Chlorine
D)Carbon monoxide
E)Nitrogen

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
The following experiment was carried out using a newly synthesized chlorofluorocarbon.Exactly 50 mL of the gas effused through a porous barrier in 172 s.The same volume of argon effused in 76 s under the same conditions.Which compound is the chlorofluorocarbon?
A)C2Cl2F4
B)C2Cl4F2
C)C2Cl3F3
D)C2Cl5F
E)C2ClF5
A)C2Cl2F4
B)C2Cl4F2
C)C2Cl3F3
D)C2Cl5F
E)C2ClF5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
If the average speed of a water molecule at 25C is 640 ms1,what is the average speed at 100C?
A)572 ms1
B)5120 ms1
C)801 ms1
D)320 ms1
E)1280 ms1
A)572 ms1
B)5120 ms1
C)801 ms1
D)320 ms1
E)1280 ms1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
What mass of aluminum metal is required to produce 12.5 L of hydrogen gas at exactly 25C and 1.00 atm? 2Al(s)+ 6H+(aq) 2Al3+(aq)+ 3H2(g)
A)20.7 g
B)22.6 g
C)10.0 g
D)13.8 g
E)9.19 g
A)20.7 g
B)22.6 g
C)10.0 g
D)13.8 g
E)9.19 g

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the mass of sulfur hexafluoride in a 2.00 L container at a pressure of 4.00 atm and a temperature of 78.0C.The molar mass of sulfur hexafluoride is 146.1 gmol1.
A)0.401 g
B)40.6 g
C)73.1 g
D)36.6 g
E)183 g
A)0.401 g
B)40.6 g
C)73.1 g
D)36.6 g
E)183 g

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Sulfur dioxide reacts with oxygen gas to produce sulfur trioxide.What volume of oxygen gas will react with 15.0 L of sulfur dioxide if both gases are at 101.3 kPa and 125C?
A)30.0 L
B)15.0 L
C)7.50 L
D)5.00 L
E)3.75 L
A)30.0 L
B)15.0 L
C)7.50 L
D)5.00 L
E)3.75 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
What is the root mean square speed of carbon dioxide molecules at 98C?
A)153 ms1
B)459 ms1
C)45.6 ms1
D)574 ms1
E)236 ms1
A)153 ms1
B)459 ms1
C)45.6 ms1
D)574 ms1
E)236 ms1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
The main composition of dry air at sea level is about 75.5% nitrogen,23.1% oxygen,and 1.3% argon.In a 1.00-g sample of dry air at 1.00 atm,calculate the partial pressure of argon gas.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
A mixture of oxygen and helium is 92.3% by mass oxygen.What is the partial pressure of oxygen if atmospheric pressure is 745 Torr?
A)412 Torr
B)446 Torr
C)688 Torr
D)333 Torr
E)299 Torr
A)412 Torr
B)446 Torr
C)688 Torr
D)333 Torr
E)299 Torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Oxygen can be produced in the laboratory by the reaction: 2KClO3(s) 2KCl(s)+ 3O2(g)
How many moles of potassium chlorate are needed to produce 257 mL of oxygen,collected over water at 14C and 97.6 kPa? The vapor pressure of water at 14C is
1.60 kPa.
A)6.89 103
B)7.00 103
C)7.12 103
D)2.06 103
E)1.55 103
How many moles of potassium chlorate are needed to produce 257 mL of oxygen,collected over water at 14C and 97.6 kPa? The vapor pressure of water at 14C is
1.60 kPa.
A)6.89 103
B)7.00 103
C)7.12 103
D)2.06 103
E)1.55 103

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
What mass of aluminum metal is required to produce 4.48 L of hydrogen gas at exactly 0C and 5.00 atm by reaction of the metal with excess strong acid?
A)1.80 g
B)9.00 g
C)36.0 g
D)18.0 g
E)180 g
A)1.80 g
B)9.00 g
C)36.0 g
D)18.0 g
E)180 g

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
If it takes 15 s for a certain sample of neon to effuse through a porous barrier,it will take ________________________________ (15 s,greater than 15 s,less than 15 s)for the same amount of nitrogen gas to effuse through the barrier under the same conditions.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
If the average speed of a carbon dioxide molecule is 410 ms1 at 25C,what is the average speed of a molecule of methane at the same temperature?
A)680 ms1
B)410 ms1
C)1130 ms1
D)1000 ms1
E)247 ms1
A)680 ms1
B)410 ms1
C)1130 ms1
D)1000 ms1
E)247 ms1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
Oxygen can be produced in the laboratory by the following reaction: 2KClO3(s) 2KCl(s)+ 3O2(g)
How many moles of potassium chlorate are needed to produce 2.75 L of oxygen,collected over water at 37C and 94.9 kPa? The vapor pressure of water at 37C is
6.28 kPa.
A)0.142
B)6.75 102
C)7.20 102
D)6.30 102
E)0.189
How many moles of potassium chlorate are needed to produce 2.75 L of oxygen,collected over water at 37C and 94.9 kPa? The vapor pressure of water at 37C is
6.28 kPa.
A)0.142
B)6.75 102
C)7.20 102
D)6.30 102
E)0.189

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following gases will have the largest root mean square speed at 100C?
A)water
B)argon
C)methane
D)nitrogen
E)oxygen
A)water
B)argon
C)methane
D)nitrogen
E)oxygen

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Lithium metal reacts with nitrogen gas to produce lithium nitride.What volume of nitrogen gas at 2.00 atm and 175C is required to produce 75.0 g of lithium nitride?
A)30.9 L
B)119 L
C)79.2 L
D)39.6 L
E)15.5 L
A)30.9 L
B)119 L
C)79.2 L
D)39.6 L
E)15.5 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
How many liters of hydrogen gas measured at 25.0C and 745 Torr are produced by detonation of 59.0 g of TNT,trinitrotoluene,C7H5(NO2)3?
A)0.544 L
B)6.94 L
C)1.36 L
D)16.2 L
A)0.544 L
B)6.94 L
C)1.36 L
D)16.2 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
Consider two cylinders of gas,both with a volume of 25 L and at 1 atm and 25C.If one cylinder contains nitrogen gas and the other argon,which of the following statements are true and which are false.
(a)The temperature of the gases is different.
(b)The average molecular speed of the gases is the same.
(c)The average kinetic energy of the gases is the same.
(a)The temperature of the gases is different.
(b)The average molecular speed of the gases is the same.
(c)The average kinetic energy of the gases is the same.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
Consider two flasks at 25C,one contains an ideal gas and the other contains the real gas SO3.Which statement regarding these gases is true?
A)After the temperature is increased about 100 K, the pressure of the ideal gas will be smaller than the pressure of SO3 because the van der Waals coefficient a for SO3 is large.
B)As the temperature is decreased, the ideal gas will liquefy first because ideal gases have larger values of the van der Waals coefficient b.
C)After the temperature has been lowered about 100 K, the pressure of the ideal gas will be smaller than the pressure of SO3 because the van der Waals coefficient a for SO3 is large.
D)As the temperature approaches 0 K, the volume of the ideal gas will be larger than the volume of SO3 because ideal gases lack intermolecular forces.
E)As the temperature approaches 0 K, the volume of the ideal gas will be smaller than the volume of SO3 because ideal gases have larger values of the van der Waals coefficient a.
A)After the temperature is increased about 100 K, the pressure of the ideal gas will be smaller than the pressure of SO3 because the van der Waals coefficient a for SO3 is large.
B)As the temperature is decreased, the ideal gas will liquefy first because ideal gases have larger values of the van der Waals coefficient b.
C)After the temperature has been lowered about 100 K, the pressure of the ideal gas will be smaller than the pressure of SO3 because the van der Waals coefficient a for SO3 is large.
D)As the temperature approaches 0 K, the volume of the ideal gas will be larger than the volume of SO3 because ideal gases lack intermolecular forces.
E)As the temperature approaches 0 K, the volume of the ideal gas will be smaller than the volume of SO3 because ideal gases have larger values of the van der Waals coefficient a.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
A plot of the Maxwell distribution against speed for different molecules shows that
A)heavy molecules have a higher average speed.
B)light molecules have a very narrow range of speeds.
C)heavy molecules have a wide range of speeds.
D)light molecules have a lower average speed.
E)heavy molecules travel with speeds close to their average values.
A)heavy molecules have a higher average speed.
B)light molecules have a very narrow range of speeds.
C)heavy molecules have a wide range of speeds.
D)light molecules have a lower average speed.
E)heavy molecules travel with speeds close to their average values.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
Ideal gases and real gases behave most similarly under which of the following conditions?
A)Low temperatures and low pressures.
B)Low temperatures and high pressures.
C)High pressures and low molar masses.
D)High temperatures and high pressures.
E)High temperatures and low pressures.
A)Low temperatures and low pressures.
B)Low temperatures and high pressures.
C)High pressures and low molar masses.
D)High temperatures and high pressures.
E)High temperatures and low pressures.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
Helium,H2,and neon all have very small values of the van der Waals coefficient "a",whereas Cl2,xenon,and water have large values.Which gases would you expect to be able to be liquefied by expansion?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the following van der Waals coefficients: 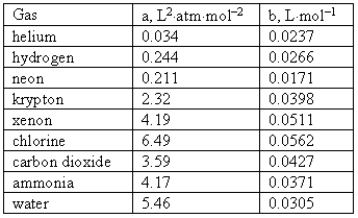 Which of the following gases has the smallest attractive forces?
Which of the following gases has the smallest attractive forces?
A)Ammonia
B)Hydrogen
C)Neon
D)Helium
E)Chlorine
 Which of the following gases has the smallest attractive forces?
Which of the following gases has the smallest attractive forces?A)Ammonia
B)Hydrogen
C)Neon
D)Helium
E)Chlorine

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
A 1.00-L sample of C2H4(g)at 2.00 atm and 293 K is burned in 8.00 L of oxygen gas at the same temperature and pressure to form carbon dioxide and water.If the reaction goes to completion,what is the final volume of all gases at 2.00 atm and 293 K?
A)5.33 L
B)9.00 L
C)8.00 L
D)3.00 L
E)5.00 L
A)5.33 L
B)9.00 L
C)8.00 L
D)3.00 L
E)5.00 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
A 6.00-L sample of C2H4(g)at 2.00 atm and 293 K is burned in 6.00 L of oxygen gas at the same temperature and pressure to form carbon dioxide and water.If the reaction goes to completion,what is the final volume of all gases at 2.00 atm and 293 K?
A)2.66 L
B)6.00 L
C)2.00 L
D)1.33 L
E)4.00 L
A)2.66 L
B)6.00 L
C)2.00 L
D)1.33 L
E)4.00 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following statements:1.Real gases act more like ideal gases as the temperature increases.2.When n and T are constant,a decrease in P results in a decrease in V.3.At 1 atm and 273 K,every molecule in a sample of a gas has the same speed.4.At constant T,CO2 molecules at 1 atm and H2 molecules at 5 atm have the same average kinetic energy.
Which of these statements is true?
A)2 and 2
B)1 and 1
C)1 and 3
D)3 and 3
E)2 and 3
Which of these statements is true?
A)2 and 2
B)1 and 1
C)1 and 3
D)3 and 3
E)2 and 3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
How many liters of nitrogen gas measured at 25.0C and 745 Torr are produced by detonation of 90.8 g of TNT,trinitrotoluene,C7H5(NO2)3?
A)0.839 L
B)1.26 L
C)9.98 L
D)15.0 L
A)0.839 L
B)1.26 L
C)9.98 L
D)15.0 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
Which molecules of the following gases will have the greatest average kinetic energy?
A)CO2 at 1 atm and 298 K
B)N2 at 1 atm and 298 K
C)All the molecules have the same kinetic energy.
D)He at 0.1 atm and 298 K
E)H2 at 0.5 atm and 298 K
A)CO2 at 1 atm and 298 K
B)N2 at 1 atm and 298 K
C)All the molecules have the same kinetic energy.
D)He at 0.1 atm and 298 K
E)H2 at 0.5 atm and 298 K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
Which molecules of the following gases will have the greatest root mean square speed?
A)Nitrogen at 1 atm and 273 K
B)All the molecules have the same root mean square speed.
C)Hydrogen at 0.5 atm and 273 K
D)Argon at 1 atm and 273 K
E)Helium at 0.1 atm and 273 K
A)Nitrogen at 1 atm and 273 K
B)All the molecules have the same root mean square speed.
C)Hydrogen at 0.5 atm and 273 K
D)Argon at 1 atm and 273 K
E)Helium at 0.1 atm and 273 K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following gases would you predict to have the largest value of the van der Waals coefficient b?
A)C2F2Cl2
B)C2FCl5
C)C2F4
D)Cl2
E)F2
A)C2F2Cl2
B)C2FCl5
C)C2F4
D)Cl2
E)F2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following gases would not be expected to cool as it expands?
A)NH3
B)Cl2
C)Xe
D)H2O
E)He
A)NH3
B)Cl2
C)Xe
D)H2O
E)He

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the following van der Waals coefficients: 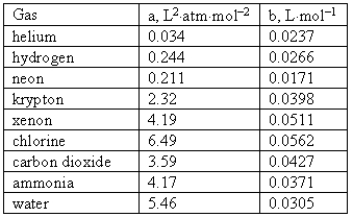 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?
A)Neon
B)Ammonia
C)Chlorine
D)Water
E)Helium
 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?A)Neon
B)Ammonia
C)Chlorine
D)Water
E)Helium

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
What is the molar volume of a nitrogen gas at 27.0oC and 1 atm?
A)2.22 L
B)22.4 L
C)24.6 L
D)20.2 L
A)2.22 L
B)22.4 L
C)24.6 L
D)20.2 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
How many liters of carbon dioxide measured at 25.0C and 821 Torr would be produced by the combustion of 319 g of glucose,C6H12O6?
A)3.37 L
B)40.1 L
C)241 L
D)20.2 L
A)3.37 L
B)40.1 L
C)241 L
D)20.2 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
What is the vapor pressure of geraniol,molar mass 155 g/mol,if the density of the vapor at 260C is 0.480 g/L?
A)50.2 Torr
B)103 Torr
C)0.0661 Torr
D)0.103 Torr
A)50.2 Torr
B)103 Torr
C)0.0661 Torr
D)0.103 Torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
A plot of the Maxwell distribution for the same gas against temperature shows that
A)at high temperatures, most molecules have speeds close to their average speed.
B)as the temperature increases, a high proportion of molecules have very slow speeds.
C)as the temperature decreases, the spread of speeds widens.
D)as the temperature decreases, a high proportion of molecules have very high speeds.
E)at low temperatures, most molecules have speeds close to their average speed.
A)at high temperatures, most molecules have speeds close to their average speed.
B)as the temperature increases, a high proportion of molecules have very slow speeds.
C)as the temperature decreases, the spread of speeds widens.
D)as the temperature decreases, a high proportion of molecules have very high speeds.
E)at low temperatures, most molecules have speeds close to their average speed.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


