Deck 13: Aqueous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 13: Aqueous Equilibria
1
What is the pH of an aqueous solution that is 0.12 M C6H5NH2 (Kb = 4.3 1010)and 0.018 M C6H5NH3Cl?
A)5.46
B)10.19
C)4.63
D)3.81
E)8.54
A)5.46
B)10.19
C)4.63
D)3.81
E)8.54
5.46
2
A solution is prepared by mixing equal volumes of 0.40 M HF(aq)with 0.40 M KOH(aq).This solution is a buffer.True or false?
False
3
What is the pH of an aqueous solution that is 0.60 (CH3)3N (Kb = 6.5 105)and 0.95 M (CH3)3NHCl?
A)4.39
B)10.01
C)3.99
D)9.81
E)9.61
A)4.39
B)10.01
C)3.99
D)9.81
E)9.61
9.61
4
What is the pH of an aqueous solution that is 0.011 M HF (Ka = 3.5 104)and 0.015 M NaF?
A)1.95
B)3.46
C)3.59
D)5.27
E)3.33
A)1.95
B)3.46
C)3.59
D)5.27
E)3.33

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the [H+] in an aqueous solution that is 0.0755 M HF and 0.100 M NaF.The value of Ka for HF is 3.5 104.
A)4.6 104 M
B)2.6 104 M
C)3.5 104 M
D)0.176 M
E)0.0755 M
A)4.6 104 M
B)2.6 104 M
C)3.5 104 M
D)0.176 M
E)0.0755 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
When pyridinium chloride is added to C5H5N(aq),
A)the pH of the solution does not change.
B)the pH of the solution increases.
C)the pH of the solution decreases.
D)the Kb increases.
E)the equilibrium concentration of NH3(aq)decreases.
A)the pH of the solution does not change.
B)the pH of the solution increases.
C)the pH of the solution decreases.
D)the Kb increases.
E)the equilibrium concentration of NH3(aq)decreases.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
The pH of 0.30 M CH3NH2(aq)is 12.0.Therefore,the pH of a solution that is 0.30 M CH3NH2(aq)and 0.10 M CH3NH3Cl(aq)is greater than 12.0.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Choose the effective pH range of a HF/NaF buffer.For HF,Ka = 3.5 104.
A)6.0-8.0
B)9.6-11.6
C)5.0-7.0
D)0.7-2.7
E)2.5-4.5
A)6.0-8.0
B)9.6-11.6
C)5.0-7.0
D)0.7-2.7
E)2.5-4.5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Choose the effective pH range of a pyridine/pyridinium chloride buffer? For pyridine,the value of Kb is 1.8 109.
A)9.1-11.1
B)1.4-3.4
C)10.3-12.3
D)7.7-9.7
E)4.3-6.3
A)9.1-11.1
B)1.4-3.4
C)10.3-12.3
D)7.7-9.7
E)4.3-6.3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
What is the pH of an aqueous solution that is 0.10 M HCOOH (Ka =1.8 104)and 0.10 M NaHCO2?
A)10.26
B)3.74
C)5.74
D)2.38
E)5.62
A)10.26
B)3.74
C)5.74
D)2.38
E)5.62

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
The pH of 0.50 M HNO2(aq)is 1.8.Therefore,the pH of a solution that is 0.50 M HNO2(aq)and 0.10 M KNO2(aq)is greater than 1.8.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
What is the pH of an aqueous solution that is 0.018 M C6H5NH2 (Kb = 4.3 1010)and 0.12 M C6H5NH3Cl?
A)5.46
B)4.63
C)3.81
D)10.19
E)8.54
A)5.46
B)4.63
C)3.81
D)10.19
E)8.54

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
When sodium nitrite is added to HNO2(aq),
A)the equilibrium concentration of HCOOH(aq)decreases.
B)the pH of the solution increases.
C)the Ka increases.
D)the pH of the solution does not change.
E)the pH of the solution decreases.
A)the equilibrium concentration of HCOOH(aq)decreases.
B)the pH of the solution increases.
C)the Ka increases.
D)the pH of the solution does not change.
E)the pH of the solution decreases.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Choose the effective pH range of an aniline/anilinium chloride buffer.The value of the Kb for aniline is 4.3 1010.
A)3.6-5.6
B)8.4-10.4
C)1.1-3.1
D)5.1-7.1
E)10.1-12.1
A)3.6-5.6
B)8.4-10.4
C)1.1-3.1
D)5.1-7.1
E)10.1-12.1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the [OH] in an aqueous solution that is 0.125 M NH3 and 0.125 M NH4Cl.The value of Kb for NH3 is 1.8 105.
A)1.8 105 M
B)5.5 1010 M
C)6.7 1012 M
D)0.125 M
E)1.5 103 M
A)1.8 105 M
B)5.5 1010 M
C)6.7 1012 M
D)0.125 M
E)1.5 103 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
What is the main factor that directly determines the pH of any buffer?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
What is the pH of an aqueous solution that is 1.0 M HClO (Ka = 3.0 108)and 0.75 M NaClO?
A)7.64
B)7.40
C)6.36
D)7.52
E)6.60
A)7.64
B)7.40
C)6.36
D)7.52
E)6.60

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
Calculate the [OH] in an aqueous solution that is 0.125 M NH3 and 0.300 M NH4Cl.The value of Kb for NH3 is 1.8 105.
A)0.425 M
B)0.125 M
C)1.8 105 M
D)7.5 106 M
E)4.3 105 M
A)0.425 M
B)0.125 M
C)1.8 105 M
D)7.5 106 M
E)4.3 105 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
What is the pH of an aqueous solution that is 0.20 M HNO2 (Ka = 4.3 104)and 0.20 M NaNO2?
A)3.67
B)2.37
C)3.37
D)4.39
E)10.63
A)3.67
B)2.37
C)3.37
D)4.39
E)10.63

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
A solution is prepared by mixing equal volumes of 0.40 M HF(aq)with 0.20 M KOH(aq).This solution is a buffer.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
The following compounds are available as 0.10 M aqueous solutions. 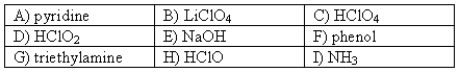 Which two solutions could be used to prepare a buffer with a pH of ~ 9? More than one answer may be possible.
Which two solutions could be used to prepare a buffer with a pH of ~ 9? More than one answer may be possible.
 Which two solutions could be used to prepare a buffer with a pH of ~ 9? More than one answer may be possible.
Which two solutions could be used to prepare a buffer with a pH of ~ 9? More than one answer may be possible.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
A buffer solution contains 0.0200 M acetic acid and 0.0200 M sodium acetate.What is the pH after 2.0 mmol of HCl is added to 1.00 L of this buffer? pKa = 4.75 for acetic acid.
A)4.70
B)4.84
C)4.75
D)4.80
E)4.66
A)4.70
B)4.84
C)4.75
D)4.80
E)4.66

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
For HF,pKa = 3.45.What is the pH of an aqueous buffer solution that is 0.100 M HF(aq)and 0.300 M KF(aq)?
A)10.07
B)2.97
C)3.45
D)3.93
E)11.03
A)10.07
B)2.97
C)3.45
D)3.93
E)11.03

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
A buffer solution contains 0.75 mol KH2PO4 and 0.75 mol K2HPO4.What is the pH after 0.10 mol KOH is added to 1.00 L of this buffer? The pKa of H2PO4 is 7.21.
A)6.91
B)6.67
C)7.21
D)7.33
E)7.09
A)6.91
B)6.67
C)7.21
D)7.33
E)7.09

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following mixtures gives a buffer with a pH greater than 7.0? For HCNO,Ka = 2.2 104 and for NH3,Kb = 1.8 105.
A)10 mL of 0.1 M NH3(aq)+ 10 mL of 0.1 M HCl(aq)
B)10 mL of 0.1 M HCNO(aq)+ 10 mL 0f 0.1 M NaOH(aq)
C)10 mL of 0.1 M HCNO(aq)+ 5.0 mL of 0.1 M NaOH(aq)
D)10 mL of 0.1 M NH3(aq)+ 10 ml of 0.1 M HCNO(aq)
E)10 mL of 0.1 M NH3(aq)+ 5.0 mL of 0.1 M HCl(aq)
A)10 mL of 0.1 M NH3(aq)+ 10 mL of 0.1 M HCl(aq)
B)10 mL of 0.1 M HCNO(aq)+ 10 mL 0f 0.1 M NaOH(aq)
C)10 mL of 0.1 M HCNO(aq)+ 5.0 mL of 0.1 M NaOH(aq)
D)10 mL of 0.1 M NH3(aq)+ 10 ml of 0.1 M HCNO(aq)
E)10 mL of 0.1 M NH3(aq)+ 5.0 mL of 0.1 M HCl(aq)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
If a small amount of a strong acid is added to buffer made up of a weak acid,HA,and the sodium salt of its conjugate base,NaA,the pH of the buffer solution does not change appreciably because
A)the Ka of HA is changed.
B)the strong acid reacts with A to give HA, which is a weak acid.
C)no reaction occurs.
D)the strong acid reacts with HA to give H2A+.
E)the strong acid reacts with A to give H2A+.
A)the Ka of HA is changed.
B)the strong acid reacts with A to give HA, which is a weak acid.
C)no reaction occurs.
D)the strong acid reacts with HA to give H2A+.
E)the strong acid reacts with A to give H2A+.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the ratio of the molarities of HPO42 and H2PO4 ions required to achieve buffering at pH = 7.00.For H3PO4,pKa1 = 2.12,pKa2 = 7.21,and pKa3 = 12.68.
A)0.81
B)1.23
C)0.62
D)0.21
E)1.62
A)0.81
B)1.23
C)0.62
D)0.21
E)1.62

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
The following compounds are available as 0.10 M aqueous solutions. 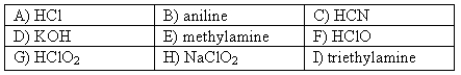 Pick two solutions that could be used to prepare a buffer with a pH of ~ 10.8.More than one answer may be possible.
Pick two solutions that could be used to prepare a buffer with a pH of ~ 10.8.More than one answer may be possible.
 Pick two solutions that could be used to prepare a buffer with a pH of ~ 10.8.More than one answer may be possible.
Pick two solutions that could be used to prepare a buffer with a pH of ~ 10.8.More than one answer may be possible.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
The following compounds are available as 0.10 M aqueous solutions. 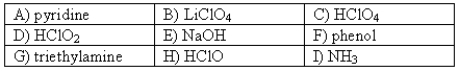 Which two solutions could be used to prepare a buffer with a pH of ~ 7?
Which two solutions could be used to prepare a buffer with a pH of ~ 7?
 Which two solutions could be used to prepare a buffer with a pH of ~ 7?
Which two solutions could be used to prepare a buffer with a pH of ~ 7?
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
The following compounds are available as 0.10 M aqueous solutions. 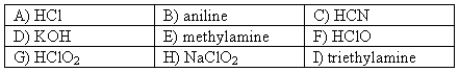 Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
 Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
For NH3,pKb = 4.74.What is the pH of an aqueous buffer solution that is 0.050 M NH3(aq)and 0.20 M NH4Cl(aq)?
A)9.86
B)5.34
C)9.26
D)8.66
E)4.14
A)9.86
B)5.34
C)9.26
D)8.66
E)4.14

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
If a small amount of a strong base is added to buffer made up of a weak acid,HA,and the sodium salt of its conjugate base,NaA,the pH of the buffer solution does not change appreciably because
A)the Ka of HA is changed.
B)No reaction occurs.
C)the strong base reacts with A to give HA, which is a weak acid.
D)the strong base reacts with HA to give AOH and H+.
E)the strong base reacts with HA to give A which is a weak base.
A)the Ka of HA is changed.
B)No reaction occurs.
C)the strong base reacts with A to give HA, which is a weak acid.
D)the strong base reacts with HA to give AOH and H+.
E)the strong base reacts with HA to give A which is a weak base.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the ratio of the molarities of CO32 and HCO3 ions required to achieve buffering at pH = 9.0.For H2CO3,pKa1 = 6.37,and pKa2 = 10.00.
A)0.50
B)3.16
C)1.65
D)0.32
E)0.61
A)0.50
B)3.16
C)1.65
D)0.32
E)0.61

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
A buffer solution contains 0.25 M NaNO2(aq)and 0.80 M HNO2(aq)(pKa = 3.37).What is the pH after 0.10 mol HBr are added to 1.00 L of this buffer?
A)11.41
B)4.15
C)2.59
D)9.85
E)3.37
A)11.41
B)4.15
C)2.59
D)9.85
E)3.37

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
The following compounds are available as 0.10 M aqueous solutions. 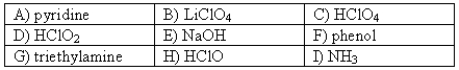 Which two solutions could be used to prepare a buffer with a pH of ~ 2.5?
Which two solutions could be used to prepare a buffer with a pH of ~ 2.5?
 Which two solutions could be used to prepare a buffer with a pH of ~ 2.5?
Which two solutions could be used to prepare a buffer with a pH of ~ 2.5?
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
For pyridine,pKb = 8.75.What is the pH of an aqueous buffer solution that is 0.300 M C5H5N(aq)and 0.500 M C5H5NHCl(aq)?
A)8.53
B)5.25
C)8.97
D)5.47
E)5.03
A)8.53
B)5.25
C)8.97
D)5.47
E)5.03

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
A buffer contains equal concentrations of a weak acid,HA,and its conjugate base,A.If the value of Ka for HA is 1.0 109,what is the pH of the buffer?
A)13.0
B)5.0
C)7.0
D)1.0
E)9.0
A)13.0
B)5.0
C)7.0
D)1.0
E)9.0

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following mixtures gives a buffer with a pH less than 7.0? For acetic acid,Ka = 1.8 105 and for NH3,Kb = 1.8 105.
A)10 mL of 0.1 M NH3(aq)+ 10 ml of 0.1 M HCl(aq)
B)10 mL of 0.1 M aqueous acetic acid + 5.0 mL of 0.1 M NaOH(aq)
C)10 mL of 0.1 M aqueous acetic acid + 10 mL of 0.1 M NaOH(aq)
D)10 mL of 0.1 M aqueous acetic acid + 10 mL 0f 0.1 M NH3(aq)
E)10 mL of 0.1 M NH3(aq)+ 5.0 mL of 0.1 M HCl(aq)
A)10 mL of 0.1 M NH3(aq)+ 10 ml of 0.1 M HCl(aq)
B)10 mL of 0.1 M aqueous acetic acid + 5.0 mL of 0.1 M NaOH(aq)
C)10 mL of 0.1 M aqueous acetic acid + 10 mL of 0.1 M NaOH(aq)
D)10 mL of 0.1 M aqueous acetic acid + 10 mL 0f 0.1 M NH3(aq)
E)10 mL of 0.1 M NH3(aq)+ 5.0 mL of 0.1 M HCl(aq)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
A buffer contains equal concentrations of NH3(aq)and NH4Cl(aq).What is the pH of the buffer? (Kb (NH3)= 1.8 105)
A)9.26
B)4.74
C)7.00
D)13.00
A)9.26
B)4.74
C)7.00
D)13.00

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
A buffer solution contains 0.0200 M acetic acid and 0.0200 M sodium acetate.What is the pH after 2.0 mmol of NaOH are added to 1.00 L of this buffer? pKa = 4.75 for acetic acid.
A)4.75
B)4.70
C)4.80
D)4.84
E)4.66
A)4.75
B)4.70
C)4.80
D)4.84
E)4.66

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
The titration curve for the titration of 0.100 M H2SO3(aq)with 0.100 M KOH(aq)is given below. 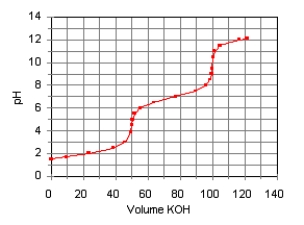 Estimate pKa1 and pKa2 of H2SO3.
Estimate pKa1 and pKa2 of H2SO3.
 Estimate pKa1 and pKa2 of H2SO3.
Estimate pKa1 and pKa2 of H2SO3.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the titration of 50.0 mL of 0.0200 M HClO(aq)with 0.100 M NaOH(aq).What is the formula of the main species in the solution after the addition of 10.0 mL of base?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
What is the pH at the stoichiometric point for the titration of 0.100 M CH3COOH(aq)with 0.100 M KOH(aq)? The value of Ka for acetic acid is 1.8 105.
A)5.28
B)8.72
C)7.00
D)9.26
E)8.89
A)5.28
B)8.72
C)7.00
D)9.26
E)8.89

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the titration of 50.0 mL of 0.0200 M C6H5COOH(aq),with 0.100 M NaOH(aq).What is the formula of the main species in the solution after the addition of 10.0 mL of base? Do not consider spectator ions.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the equilibrium constant for the reaction that occurs when sodium hydroxide is added to the buffer B(aq)/BHCl(aq).The Kb of B is 1.5 105.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the equilibrium constant for the reaction that occurs when perchloric acid is added to the buffer B(aq)/BHCl(aq).The Kb of B is 3.4 105.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the equilibrium constant for the reaction that occurs when nitric acid is added to the buffer HA(aq)/NaA(aq).The Ka of HA is 1.2 105.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
What is the pH at the half-stoichiometric point for the titration of 0.22 M HNO2(aq)with 0.10 M KOH(aq)? For HNO2,Ka = 4.3 104.
A)2.31
B)7.00
C)2.01
D)3.37
E)2.16
A)2.31
B)7.00
C)2.01
D)3.37
E)2.16

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following indicators would be most suitable for the titration of 0.10 M (CH3)3N(aq)with 0.10 M HClO4(aq)? For trimethyamine,pKb = 4.19.
A)Bromothymol blue (pKIn = 7.1)
B)Alizarin yellow (pKIn = 11.2)
C)Bromocresol green (pKIn = 4.7)
D)Tthymol blue (pKIn = 1.7)
E)Phenolphthalein (pKIn = 9.4)
A)Bromothymol blue (pKIn = 7.1)
B)Alizarin yellow (pKIn = 11.2)
C)Bromocresol green (pKIn = 4.7)
D)Tthymol blue (pKIn = 1.7)
E)Phenolphthalein (pKIn = 9.4)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
The curve for the titration of 50.0 mL of 0.0200 M C6H5COOH(aq)with 0.100 M NaOH(aq)is given below.What are the main species in the solution after 7.5 mL of base have been added? 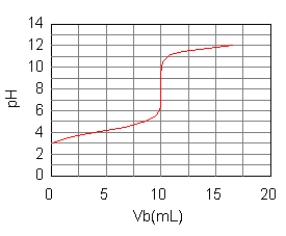


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the titration of 10.0 mL of 0.100 M (CH3)3N(aq)with 0.100 M HClO4(aq).What is the formula of the main species in the solution after the addition of 10.0 mL of acid?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pH at the stoichiometric point for the titration of 0.26 M CH3NH2(aq)with 0.26 M HClO4(aq)? For CH3NH2,Kb = 3.6 104.
A)5.72
B)7.00
C)5.57
D)2.16
E)2.01
A)5.72
B)7.00
C)5.57
D)2.16
E)2.01

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
What is the pH at the half-stoichiometric point for the titration of 0.88 M HNO2(aq)with 0.10 M KOH(aq)? For HNO2,Ka = 4.3 104.
A)3.37
B)2.01
C)1.86
D)7.00
E)1.71
A)3.37
B)2.01
C)1.86
D)7.00
E)1.71

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following indicators would be most suitable for the titration of 0.10 M lactic acid with 0.10 M KOH(aq)? For lactic acid,pKa = 3.08.
A)Methyl orange (pKIn = 3.4)
B)Thymol blue (pKIn = 1.7)
C)Alizarin yellow (pKIn = 11.2)
D)Bromophenol blue (pKIn = 3.9)
E)Phenol red (pKIn = 7.9)
A)Methyl orange (pKIn = 3.4)
B)Thymol blue (pKIn = 1.7)
C)Alizarin yellow (pKIn = 11.2)
D)Bromophenol blue (pKIn = 3.9)
E)Phenol red (pKIn = 7.9)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
At the stoichiometric point in the titration of 0.130 M HCOOH(aq)with 0.130 M KOH(aq),
A)the pH is 7.0.
B)[HCOOH] = 0.0650 M.
C)[HCO2] = 0.130 M.
D)the pH is greater than 7.
E)the pH is less than 7.
A)the pH is 7.0.
B)[HCOOH] = 0.0650 M.
C)[HCO2] = 0.130 M.
D)the pH is greater than 7.
E)the pH is less than 7.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
What is the concentration of acetate ion at the stoichiometric point in the titration of 0.018 M CH3COOH(aq)with 0.036 M NaOH(aq)? For acetic acid,Ka = 1.8 105.
A)0.018 M
B)0.0090 M
C)0.024 M
D)0.012 M
E)0.036 M
A)0.018 M
B)0.0090 M
C)0.024 M
D)0.012 M
E)0.036 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the equilibrium constant for the reaction that occurs when sodium hydroxide is added to the buffer HA(aq)/NaA(aq).The Ka of HA is 4.1 105.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
For the titration of 50.0 mL of 0.020 M aqueous salicylic acid with 0.020 M KOH(aq),calculate the pH after the addition of 55.0 mL of KOH(aq).For salycylic acid,pKa = 2.97.
A)10.98
B)7.00
C)11.26
D)12.02
E)12.30
A)10.98
B)7.00
C)11.26
D)12.02
E)12.30

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
At the stoichiometric point in the titration of 0.260 M CH3NH2(aq)with 0.260 M HCl(aq),
A)the pH is less than 7.
B)[CH3NH3+] = 0.260 M.
C)the pH is 7.0.
D)[CH3NH2] = 0.130 M.
E)the pH is greater than 7.
A)the pH is less than 7.
B)[CH3NH3+] = 0.260 M.
C)the pH is 7.0.
D)[CH3NH2] = 0.130 M.
E)the pH is greater than 7.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
What is the concentration of acetate ion at the stoichiometric point in the titration of 0.018 M CH3COOH(aq)with 0.072 M NaOH(aq)? For acetic acid,Ka = 1.8 105.
A)0.054 M
B)0.036 M
C)0.018 M
D)0.072 M
E)0.014 M
A)0.054 M
B)0.036 M
C)0.018 M
D)0.072 M
E)0.014 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
The titration curve for the titration of 0.100 M H2SO3(aq)with 0.100 M KOH(aq)is given below. 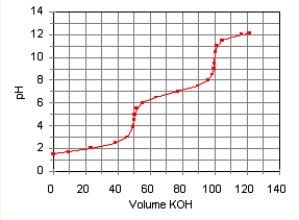 The major species in solution after 75 mL of KOH(aq)has been added are
The major species in solution after 75 mL of KOH(aq)has been added are
A)HSO3(aq)and Na+(aq).
B)SO32(aq), and Na+(aq).
C)SO32(aq), OH(aq), and Na+(aq).
D)H2SO3(aq), HSO3, and Na+(aq).
E)HSO3(aq), SO32(aq), and Na+(aq).
 The major species in solution after 75 mL of KOH(aq)has been added are
The major species in solution after 75 mL of KOH(aq)has been added areA)HSO3(aq)and Na+(aq).
B)SO32(aq), and Na+(aq).
C)SO32(aq), OH(aq), and Na+(aq).
D)H2SO3(aq), HSO3, and Na+(aq).
E)HSO3(aq), SO32(aq), and Na+(aq).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
Consider the titration of 15.0 mL of 0.200 M H3PO4(aq)with 0.200 M NaOH(aq).What is(are)the major species in solution after the addition of 30.0 mL of base?
A)HPO42(aq)
B)H2PO4(aq)
C)H2PO4(aq)and HPO42(aq)
D)H3PO4(aq)and H2PO4(aq)
E)PO43(aq)
A)HPO42(aq)
B)H2PO4(aq)
C)H2PO4(aq)and HPO42(aq)
D)H3PO4(aq)and H2PO4(aq)
E)PO43(aq)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the solubility product of calcium hydroxide given that the solubility of Ca(OH)2(s)in water at 25C is 0.011 M.
A)1.5 108
B)1.1 105
C)2.7 106
D)5.3 106
E)1.2 104
A)1.5 108
B)1.1 105
C)2.7 106
D)5.3 106
E)1.2 104

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following water-insoluble salts is more soluble in 1.0 M HClO4(aq)?
A)AgBr
B)PbF2
C)Hg2Br2
D)PbI2
E)AgClO4
A)AgBr
B)PbF2
C)Hg2Br2
D)PbI2
E)AgClO4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the titration of 15.0 mL of 0.200 M H3PO4(aq)with 0.200 M NaOH(aq).What is/are the major species in solution after the addition of 30.0 mL of base?
A)OH(aq)
B)H3PO4(aq)and H2PO4(aq)
C)HPO42(aq)
D)PO43(aq)
A)OH(aq)
B)H3PO4(aq)and H2PO4(aq)
C)HPO42(aq)
D)PO43(aq)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate the value of the equilibrium constant for the reaction AgCl(s)+ 2NH3(aq)  Ag(NH3)2+(aq)+ Cl(aq)
Ag(NH3)2+(aq)+ Cl(aq)
Given Ksp = 1.6 1010 for silver chloride and Kf = 1.6 107 for the ammonia complex of Ag+ ions,Ag(NH3)2+.
A)1.0 1017
B)6.3 109
C)6.3 108
D)1.0 1017
E)2.6 103
 Ag(NH3)2+(aq)+ Cl(aq)
Ag(NH3)2+(aq)+ Cl(aq)Given Ksp = 1.6 1010 for silver chloride and Kf = 1.6 107 for the ammonia complex of Ag+ ions,Ag(NH3)2+.
A)1.0 1017
B)6.3 109
C)6.3 108
D)1.0 1017
E)2.6 103

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
If equal volumes of 0.004 M Pb(NO3)2(aq)and 0.004 M KI(aq)are mixed,what reaction,if any,occurs? The value of Ksp for PbI2 is 1.4 108.
A)The solution turns purple because of formation of I2.
B)PbI2(s)precipitates.
C)KNO3(s)precipitates.
D)No reaction occurs.
E)The value of Ksp changes to 9 109.
A)The solution turns purple because of formation of I2.
B)PbI2(s)precipitates.
C)KNO3(s)precipitates.
D)No reaction occurs.
E)The value of Ksp changes to 9 109.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
The titration curve for the titration of 0.100 M Na2CO3(aq)with 0.100 M HClO4(aq)is: 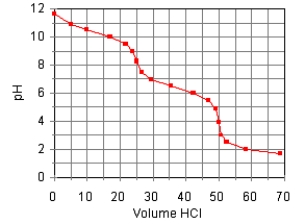 Estimate pKb2.
Estimate pKb2.
A)7.6
B)10.3
C)6.4
D)8.5
E)3.7
 Estimate pKb2.
Estimate pKb2.A)7.6
B)10.3
C)6.4
D)8.5
E)3.7

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
Silver bromide is most soluble in
A)pure H2O(l).
B)dilute HNO3(aq).
C)0.10 M AgNO3(aq).
D)dilute NH3(aq).
E)0.10 M NaCl(aq).
A)pure H2O(l).
B)dilute HNO3(aq).
C)0.10 M AgNO3(aq).
D)dilute NH3(aq).
E)0.10 M NaCl(aq).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
You have available the following reagents as 0.10 M aqueous solutions: NaOH,HCl,HCN (pKa = 9.31),aniline (pKb = 9.13),HNO2 (pKa = 3.25),and CH3NH2 (pKb = 3.34).Which two reagents would you use to make a buffer with a pH of 10.6?
A)NaOH and HCN
B)HCl and aniline
C)HCl and CH3NH2
D)NaOH and HNO2
A)NaOH and HCN
B)HCl and aniline
C)HCl and CH3NH2
D)NaOH and HNO2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
What is the equilibrium constant for the titration reaction involving CH3NH2(aq)and HBr(aq)?
A)1.0 1014
B)2.8 103
C)2.8 1011
D)3.6 1010
A)1.0 1014
B)2.8 103
C)2.8 1011
D)3.6 1010

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
What is the relationship between the solubility in water,s,and Ksp for the ionic solid Ca3(PO4)2?
A)Ksp = 72s5
B)Ksp = 5s
C)Ksp = 6s2
D)Ksp = s5
A)Ksp = 72s5
B)Ksp = 5s
C)Ksp = 6s2
D)Ksp = s5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
What is the main factor that determines the pH of any buffer?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
A certain weak acid has a Ka of 2.0 105.What is the equilibrium constant for the reaction of this acid with a strong base?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
What is the equilibrium constant for the titration reaction involving HClO4(aq)and Ba(OH)2(aq)?
A)1.0 1014
B)2.0 1014
C)1.0 107
D)1.0 1014
A)1.0 1014
B)2.0 1014
C)1.0 107
D)1.0 1014

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
If the molar solubility of the compound M2A3 is 7.0 106 M,what is the Ksp for this compound?
A)1.7 1026
B)1.8 1024
C)2.9 1010
D)3.5 105
A)1.7 1026
B)1.8 1024
C)2.9 1010
D)3.5 105

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
The titration curve for the titration of 0.100 M Na2CO3(aq)with 0.100 M HClO4(aq)is: 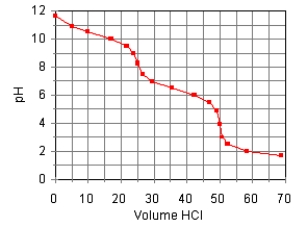 Estimate pKb1.
Estimate pKb1.
A)8.5
B)6.4
C)3.7
D)10.3
E)7.6
 Estimate pKb1.
Estimate pKb1.A)8.5
B)6.4
C)3.7
D)10.3
E)7.6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
The titration curve for the titration of 0.100 M Na2CO3(aq)with 0.100 M HClO4(aq)is: 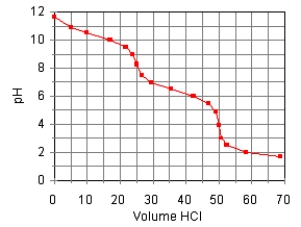 The main species in the solution after the addition of 35 mL of HClO4 are
The main species in the solution after the addition of 35 mL of HClO4 are
A)HCO3, H2CO3, Na+, and ClO4.
B)H2CO3, Na+, and ClO4.
C)CO32, HCO3, Na+, and ClO4.
D)CO32, Na+, and ClO4.
E)HCO3, Na+, and ClO4.
 The main species in the solution after the addition of 35 mL of HClO4 are
The main species in the solution after the addition of 35 mL of HClO4 areA)HCO3, H2CO3, Na+, and ClO4.
B)H2CO3, Na+, and ClO4.
C)CO32, HCO3, Na+, and ClO4.
D)CO32, Na+, and ClO4.
E)HCO3, Na+, and ClO4.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
If you wish to increase the solubility of silver benzoate,a preservative,you would
A)add sodium hydroxide.
B)decrease the pH.
C)add sodium acetate.
D)add sodium benzoate.
E)add silver nitrate.
A)add sodium hydroxide.
B)decrease the pH.
C)add sodium acetate.
D)add sodium benzoate.
E)add silver nitrate.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
The Cu2+ ion can be separated from Ag+,Ca2+,and K+ in aqueous solution by
A)precipitation of Cu2+ as CuS(s)at pH-1.
B)precipitation of Cu2+ as Cu(OH)2(s)with 6 M NaOH(aq).
C)precipitation of Cu2+ as CuCl2(s)with 6 M HCl(aq).
D)precipitation of Ag+, Ca2+, and K+ as the carbonates.
E)None of these procedures will separate Cu2+ from the other ions.
A)precipitation of Cu2+ as CuS(s)at pH-1.
B)precipitation of Cu2+ as Cu(OH)2(s)with 6 M NaOH(aq).
C)precipitation of Cu2+ as CuCl2(s)with 6 M HCl(aq).
D)precipitation of Ag+, Ca2+, and K+ as the carbonates.
E)None of these procedures will separate Cu2+ from the other ions.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


