Deck 1: The Quantum World
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 1: The Quantum World
1
What is the wavelength of radiation with a frequency of 2.3 1013 /sec?
A)3.0 108 m
B)7.6 105 cm
C)7.6 105 m
D)1.3 10-5 cm
E)1.3 10-5 m
A)3.0 108 m
B)7.6 105 cm
C)7.6 105 m
D)1.3 10-5 cm
E)1.3 10-5 m
1.3 10-5 m
2
Which of the following statements regarding electromagnetic radiation is true?
A)Electromagnetic radiation with a wavelength of 800 nm is in the ultraviolet range.
B)Electromagnetic radiation with a wavelength of 900 nm is in the visible light range.
C)Electromagnetic radiation with a wavelength of 400 nm is in the visible light range.
D)Electromagnetic radiation with a wavelength of 600 nm is in the ultraviolet range.
E)Electromagnetic radiation with a wavelength of 600 nm is in the visible light range.
A)Electromagnetic radiation with a wavelength of 800 nm is in the ultraviolet range.
B)Electromagnetic radiation with a wavelength of 900 nm is in the visible light range.
C)Electromagnetic radiation with a wavelength of 400 nm is in the visible light range.
D)Electromagnetic radiation with a wavelength of 600 nm is in the ultraviolet range.
E)Electromagnetic radiation with a wavelength of 600 nm is in the visible light range.
Electromagnetic radiation with a wavelength of 600 nm is in the visible light range.
3
What is the wavelength of radiation with a frequency of 1.1 1011 /sec?
A)3.7 102 m
B)2.7 10-3 cm
C)2.7 10-3 m
D)3.7 102 cm
E)2.7 10-13 m
A)3.7 102 m
B)2.7 10-3 cm
C)2.7 10-3 m
D)3.7 102 cm
E)2.7 10-13 m
2.7 10-3 m
4
Radiation with a frequency of 3.91014/sec has what wavelength?
A)1.3106 m
B)7.710-7 cm
C)7.710-7 m
D)3.1106 m
E)1.3106 cm
A)1.3106 m
B)7.710-7 cm
C)7.710-7 m
D)3.1106 m
E)1.3106 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the smallest wavelength electromagnetic radiation?
A)Radio.
B)Microwave.
C)Ultraviolet.
D)Visible blue light.
E)Infrared.
A)Radio.
B)Microwave.
C)Ultraviolet.
D)Visible blue light.
E)Infrared.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
What is the wavelength of a radio station transmitting at 99.1 MHz?
A)330 nm
B)303 nm
C)0.00303 m
D)3.03 m
E)0.330 m
A)330 nm
B)303 nm
C)0.00303 m
D)3.03 m
E)0.330 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Electromagnetic radiation encompasses which of the following?
A)Microwaves.
B)Alpha particles.
C)Gamma radiation.
D)A and C above.
E)B and C above.
A)Microwaves.
B)Alpha particles.
C)Gamma radiation.
D)A and C above.
E)B and C above.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Wavelength is defined how,in terms of electromagnetic radiation?
A)The peak to peak distance between waves.
B)The peak to trough distance between waves.
C)The speed with which a wave traverses a distance.
D)The amplitude with which a wave traverses a distance.
E)The trough to peak distance between a wave as well as the speed the wave traverses this distance.
A)The peak to peak distance between waves.
B)The peak to trough distance between waves.
C)The speed with which a wave traverses a distance.
D)The amplitude with which a wave traverses a distance.
E)The trough to peak distance between a wave as well as the speed the wave traverses this distance.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
What is the frequency of yellow light with a wavelength of 580 nm?
A)5.2 1016 Hz
B)1.9 1017 Hz
C)5.2 1014 Hz
D)5.2 1012 Hz
E)1.9 1015 Hz
A)5.2 1016 Hz
B)1.9 1017 Hz
C)5.2 1014 Hz
D)5.2 1012 Hz
E)1.9 1015 Hz

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements regarding electromagnetic radiation is true?
A)Electromagnetic radiation with a wavelength of 400 nm travels faster than that with a wavelength of 600 nm.
B)The frequency of electromagnetic radiation determines how fast it travels.
C)Electromagnetic radiation with a wavelength of 400 nm has a frequency that is smaller than that with a wavelength of 600 nm.
D)Electromagnetic radiation with a wavelength of 600 nm travels faster than that with a wavelength of 400 nm.
E)Electromagnetic radiation with a wavelength of 600 nm has a frequency that is smaller than that with a wavelength of 400 nm.
A)Electromagnetic radiation with a wavelength of 400 nm travels faster than that with a wavelength of 600 nm.
B)The frequency of electromagnetic radiation determines how fast it travels.
C)Electromagnetic radiation with a wavelength of 400 nm has a frequency that is smaller than that with a wavelength of 600 nm.
D)Electromagnetic radiation with a wavelength of 600 nm travels faster than that with a wavelength of 400 nm.
E)Electromagnetic radiation with a wavelength of 600 nm has a frequency that is smaller than that with a wavelength of 400 nm.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Visible light,microwaves,and x-rays travel through empty space at 3.00 108 ms1.True or false?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Radiation with a wavelength of 2.2 mm is most likely in what part of the electromagnetic spectrum?
A)Cosmic rays.
B)X - rays.
C)Microwaves.
D)Ultraviolet radiation.
E)Gamma radiation.
A)Cosmic rays.
B)X - rays.
C)Microwaves.
D)Ultraviolet radiation.
E)Gamma radiation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
The ultraviolet range encompasses what wavelengths?
A)100 - 450nm.
B)530 - 580 nm.
C)1 - 50 nm.
D)10 - 80 nm.
E)420 - 500 nm.
A)100 - 450nm.
B)530 - 580 nm.
C)1 - 50 nm.
D)10 - 80 nm.
E)420 - 500 nm.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
When radiation has a frequency of 6.91017 Hz,what is its wavelength?
A)4.310-10 m
B)3.410-10 m
C)3.010-8 m
D)2.310-9 m
E)2.310-8 nm
A)4.310-10 m
B)3.410-10 m
C)3.010-8 m
D)2.310-9 m
E)2.310-8 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
Estimate the frequency of the wave shown below. 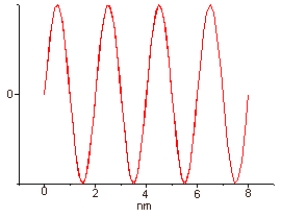
A)1.5 × 1017 s−1
B)3.0 × 1017 s−1
C)1.0 × 1017 s−1
D)2.0 × 1017 s−1
E)Not enough information is given to permit calculation of the frequency.

A)1.5 × 1017 s−1
B)3.0 × 1017 s−1
C)1.0 × 1017 s−1
D)2.0 × 1017 s−1
E)Not enough information is given to permit calculation of the frequency.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
What wavelength does radiation have when its frequency is 5.61013 /sec?
A)5.410-9 m
B)5.410-8 m
C)5.410-7 m
D)5.410-6 m
E)5.410-6 cm
A)5.410-9 m
B)5.410-8 m
C)5.410-7 m
D)5.410-6 m
E)5.410-6 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is the highest frequency electromagnetic radiation?
A)X-radiation.
B)Ultraviolet.
C)Visible light.
D)Microwave.
E)Radio.
A)X-radiation.
B)Ultraviolet.
C)Visible light.
D)Microwave.
E)Radio.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following has the highest energy per photon?
A)Infrared
B)Blue light
C)Green light
D)Orange light
E)Red light
A)Infrared
B)Blue light
C)Green light
D)Orange light
E)Red light

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Radiation with a wavelength of 2 - 10 pm is in what part of the electromagnetic spectrum?
A)Radio waves.
B)Microwave radiation.
C)Ultraviolet radiation.
D)X - radiation.
E)Gamma radiation.
A)Radio waves.
B)Microwave radiation.
C)Ultraviolet radiation.
D)X - radiation.
E)Gamma radiation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
What wavelength does radiation possess when its frequency is 2.1 1015 Hz?
A)4.1 10-7 cm
B)1.4 10-7 m
C)6.5 106 m
D)7.0 106 m
E)1.4 10-7 nm
A)4.1 10-7 cm
B)1.4 10-7 m
C)6.5 106 m
D)7.0 106 m
E)1.4 10-7 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
What is the frequency of radiation with a wavelength of 2.0 m?
A)1.5 108 /sec
B)3.0 108 /sec
C)7.0 10-9 /sec
D)7.0 108 /sec
E)1.5 10-8 /sec
A)1.5 108 /sec
B)3.0 108 /sec
C)7.0 10-9 /sec
D)7.0 108 /sec
E)1.5 10-8 /sec

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
What is the wavelength of radiation with a frequency of 2.7 1018 m?
A)1.1 10-11 cm
B)1.1 10-11 m
C)9.9 10-11 m
D)9.9 10-11 cm
E)3.0 10-11 m
A)1.1 10-11 cm
B)1.1 10-11 m
C)9.9 10-11 m
D)9.9 10-11 cm
E)3.0 10-11 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is a Lyman series line?
A)954.6 nm
B)656.3 nm
C)486.1 nm
D)434.0 nm
E)None of the above.
A)954.6 nm
B)656.3 nm
C)486.1 nm
D)434.0 nm
E)None of the above.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
What is the general wavelength of visible light?
A)1 - 100 nm
B)100 - 400 nm
C)420 - 700 nm
D)750 - 1,000 nm
E)> 1,000 nm
A)1 - 100 nm
B)100 - 400 nm
C)420 - 700 nm
D)750 - 1,000 nm
E)> 1,000 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following corresponds to an X-radiation wavelength?
A)0.0001 nm
B)0.001 nm
C)9 cm
D)9 nm
E)95 nm
A)0.0001 nm
B)0.001 nm
C)9 cm
D)9 nm
E)95 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
If radiation has a frequency of 3.1 1016 sec-1,what is its wavelength?
A)1.0 108 cm
B)1.0 10-8 cm
C)1.0 108 m
D)1.0 10-8 m
E)3.0 10-8 m
A)1.0 108 cm
B)1.0 10-8 cm
C)1.0 108 m
D)1.0 10-8 m
E)3.0 10-8 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
What magnitude of wavelength and frequency does X-radiation have?
A)Small wavelength and low frequency.
B)Small wavelength and high frequency.
C)Large wavelength and low frequency.
D)Large wavelength and high frequency.
E)Wavelength almost the same as that in visible light.
A)Small wavelength and low frequency.
B)Small wavelength and high frequency.
C)Large wavelength and low frequency.
D)Large wavelength and high frequency.
E)Wavelength almost the same as that in visible light.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following has the highest frequency?
A)Microwave radiation
B)Infrared radiation
C)Visible light
D)Ultraviolet radiation
E)X-rays
A)Microwave radiation
B)Infrared radiation
C)Visible light
D)Ultraviolet radiation
E)X-rays

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
How is gamma radiation best described?
A)As very small wavelength and low frequency.
B)As wavelength very near that of the visible spectrum.
C)As large wavelength.
D)As very low frequency.
E)As very high frequency.
A)As very small wavelength and low frequency.
B)As wavelength very near that of the visible spectrum.
C)As large wavelength.
D)As very low frequency.
E)As very high frequency.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
In 1.0 s,a 60 W bulb emits 11 J of energy in the form of infrared radiation (heat)of wavelength 1850 nm.How many photons of infrared radiation does the lamp generate in 1.0 s?
A)1.0 1029
B)1.0 1020
C)6.8 1014
D)1.1 1019
E)6.6 1023
A)1.0 1029
B)1.0 1020
C)6.8 1014
D)1.1 1019
E)6.6 1023

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Of the following,which is a Balmer series visible band?
A)954.6 nm
B)656.3 nm
C)401.2 nm
D)121.6 nm
E)97.3 nm
A)954.6 nm
B)656.3 nm
C)401.2 nm
D)121.6 nm
E)97.3 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
When radiation has a frequency of 1.5 1015 Hz,what is its wavelength?
A)3.0 10-11 m
B)4.2 10-7 m
C)2.0 10-7 cm
D)2.0 10-7 m
E)2.0 10-8 m
A)3.0 10-11 m
B)4.2 10-7 m
C)2.0 10-7 cm
D)2.0 10-7 m
E)2.0 10-8 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
What is the wavelength of radiation that has a frequency of 4.9 x 1013 /sec?
A)6.1 10-6 m
B)6.1 10-6 cm
C)6.1 106 m
D)1.6 105 m
E)1.6 10-5 m
A)6.1 10-6 m
B)6.1 10-6 cm
C)6.1 106 m
D)1.6 105 m
E)1.6 10-5 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
What is implied about an electron in an atom when it is observed that it has discrete spectral lines?
A)The electron is negatively charged.
B)The electron is oppositely charged from the proton.
C)The electron circles the nucleus.
D)The electron can have only certain energies.
E)The electron has negligible mass.
A)The electron is negatively charged.
B)The electron is oppositely charged from the proton.
C)The electron circles the nucleus.
D)The electron can have only certain energies.
E)The electron has negligible mass.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
What does the term "black body" signify for an object?
A)The object is white hot.
B)The object is black to human sight.
C)The object absorbs and emits all wavelengths
D)The object absorbs certain wavelengths of light preferentially.
E)There is no significance to the name.
A)The object is white hot.
B)The object is black to human sight.
C)The object absorbs and emits all wavelengths
D)The object absorbs certain wavelengths of light preferentially.
E)There is no significance to the name.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following has the largest wavelengths?
A)Red visible light
B)Blue visible light
C)Microwaves
D)Ultraviolet radiation
E)Gamma radiation
A)Red visible light
B)Blue visible light
C)Microwaves
D)Ultraviolet radiation
E)Gamma radiation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a Lyman series UV band?
A)121.6 nm
B)221.6 nm
C)434.0 nm
D)121.6 cm
E)410.2 nm
A)121.6 nm
B)221.6 nm
C)434.0 nm
D)121.6 cm
E)410.2 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
What type of wavelength is characteristic of radio waves?
A)Very large.
B)Very small.
C)Large or small depending on frequency.
D)Close to that of visible light.
E)None of the above; radio waves are not a form of radiation.
A)Very large.
B)Very small.
C)Large or small depending on frequency.
D)Close to that of visible light.
E)None of the above; radio waves are not a form of radiation.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Of the following,which wavelength range is visible to the human eye?
A)950 nm
B)870 nm
C)770 nm
D)500 nm
E)380 nm
A)950 nm
B)870 nm
C)770 nm
D)500 nm
E)380 nm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
When radiation has a frequency of 5.2 1012 Hz,what is its wavelength?
A)5.8 10-5 cm
B)1.7 104 m
C)1.7 10-4 m
D)5.8 10-5 m
E)5.8 105 m
A)5.8 10-5 cm
B)1.7 104 m
C)1.7 10-4 m
D)5.8 10-5 m
E)5.8 105 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
What is the energy of a mole of photons with a frequency of 2.9 1015 sec-1?
A)1.2 106J/mol
B)1.2 109J/mol
C)8.3 107J/mol
D)8.3 107J/mol
E)6.7 106J/mol
A)1.2 106J/mol
B)1.2 109J/mol
C)8.3 107J/mol
D)8.3 107J/mol
E)6.7 106J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
A photon with a frequency of 1.2 1015 Hz has what energy?
A)8.0 10-19
B)1.0 10-19
C)8.0 1019
D)5.5 1049
E)5.5 1049
A)8.0 10-19
B)1.0 10-19
C)8.0 1019
D)5.5 1049
E)5.5 1049

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
A photon with energy of 3.910-17 J has what frequency,in /sec?
A)6.6 10-34
B)3.7 1015
C)3.7 1015
D)2.7 1014
E)2.7 10-14
A)6.6 10-34
B)3.7 1015
C)3.7 1015
D)2.7 1014
E)2.7 10-14

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
When a photon has an energy of 1.3 10-20 J,what will its frequency be,in Hz?
A)1.9 1014
B)9.1 1020
C)1.9 1013
D)9.1 1019
E)6.0 1013
A)1.9 1014
B)9.1 1020
C)1.9 1013
D)9.1 1019
E)6.0 1013

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
When a mole of photons has the frequency 4.2 1015 Hz,what is its energy?
A)1.7 10-6J/mol
B)5.9 10-7J/mol
C)5.9 107J/mol
D)6.2 108J/mol
E)1.7 106J/mol
A)1.7 10-6J/mol
B)5.9 10-7J/mol
C)5.9 107J/mol
D)6.2 108J/mol
E)1.7 106J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
A photon with an energy of 1.8 10-19 J possesses what frequency in sec-1?
A)7.2 10-14
B)2.7 1015
C)2.7 1014
D)2.7 1014
E)7.2 1015
A)7.2 10-14
B)2.7 1015
C)2.7 1014
D)2.7 1014
E)7.2 1015

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
What is the frequency of a photon that has an energy of 5.4 10-18 J?
A)1.6 10-34Hz
B)1.2 1016Hz
C)1.2 1016Hz
D)8.1 1015Hz
A)1.6 10-34Hz
B)1.2 1016Hz
C)1.2 1016Hz
D)8.1 1015Hz

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
If a photon has an energy of 4.0 10-16 J,what will its frequency be,in Hz?
A)6.0 1017
B)6.0 1016
C)3.9 1017
D)1.8 1017
E)1.3 1020
A)6.0 1017
B)6.0 1016
C)3.9 1017
D)1.8 1017
E)1.3 1020

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
What is the energy of a mole of photons with a frequency of 3.8 1014 /sec?
A)1.5 105J/mol
B)1.5 10-5J/mol
C)6.7 107J/mol
D)6.7 107J/mol
E)3.8 10-5J/mol
A)1.5 105J/mol
B)1.5 10-5J/mol
C)6.7 107J/mol
D)6.7 107J/mol
E)3.8 10-5J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
What is the energy of a photon with a frequency of 3.8 1014 Hz?
A)1.5 10-15J
B)3.8 1014J
C)8.3 1014J
D)3.8 1019J
E)1.5 10-15J
A)1.5 10-15J
B)3.8 1014J
C)8.3 1014J
D)3.8 1019J
E)1.5 10-15J

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
A mole of photons with a frequency of 3.1 1014 /sec has what energy?
A)1.2 10-5J/mol
B)8.3 10-7J/mol
C)8.3 107J/mol
D)7.1 107J/mol
E)1.2 105J/mol
A)1.2 10-5J/mol
B)8.3 10-7J/mol
C)8.3 107J/mol
D)7.1 107J/mol
E)1.2 105J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
What is the energy of a mole of photons with a frequency of 6.9 1014 Hz?
A)2.8 105J/mol
B)8.3 104J/mol
C)8.3 104J/mol
D)3.8 106J/mol
E)2.8 10-6J/mol
A)2.8 105J/mol
B)8.3 104J/mol
C)8.3 104J/mol
D)3.8 106J/mol
E)2.8 10-6J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
What is the energy of a mole of photons with a frequency of 3.2 1016 Hz?
A)1.3 107J/mol
B)1.3 10-7J/mol
C)3.2 1016J/mol
D)7.7 108J/mol
E)7.7 108J/mol
A)1.3 107J/mol
B)1.3 10-7J/mol
C)3.2 1016J/mol
D)7.7 108J/mol
E)7.7 108J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
How many joules of energy does a photon have with a frequency of 6.8 1015 Hz?
A)4.5 1028
B)5.6 10-18
C)4.5 1018
D)5.6 1019
E)4.5 10-23
A)4.5 1028
B)5.6 10-18
C)4.5 1018
D)5.6 1019
E)4.5 10-23

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
A mole of photons with a frequency of 7.5 1016 Hz has what energy?
A)3.0 10-7J/mol
B)4.2 106J/mol
C)3.3 108J/mol
D)3.0 107J/mol
E)3.3 108J/mol
A)3.0 10-7J/mol
B)4.2 106J/mol
C)3.3 108J/mol
D)3.0 107J/mol
E)3.3 108J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
A photon with a frequency of 2.3 1016 Hz has how many joules of energy?
A)1.5 10-17
B)1.5 1017
C)3.5 1017
D)3.5 1017
E)3.0 108
A)1.5 10-17
B)1.5 1017
C)3.5 1017
D)3.5 1017
E)3.0 108

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
What is the energy of a mole of photons with a frequency of 1.2 1014 Hz?
A)4.8 10-4J/mol
B)3.8 104J/mol
C)4.8 104J/mol
D)3.8 104J/mol
E)8.4 105J/mol
A)4.8 10-4J/mol
B)3.8 104J/mol
C)4.8 104J/mol
D)3.8 104J/mol
E)8.4 105J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
What is the energy in joules of a photon with a frequency of 5.6 1014 Hz?
A)3.7 10-21
B)6.8 1020
C)6.8 1019
D)3.7 1019
E)3.7 1019
A)3.7 10-21
B)6.8 1020
C)6.8 1019
D)3.7 1019
E)3.7 1019

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
What energy in joules does a photon with a frequency of 5.0 1014 /sec have?
A)1.3 10-19
B)3.3 10-19
C)6.6 1019
D)6.6 1019
E)3.3 1019
A)1.3 10-19
B)3.3 10-19
C)6.6 1019
D)6.6 1019
E)3.3 1019

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
What is the frequency,in Hz,of a photon with energy of 2.3 10-17 J?
A)3.4 10-16
B)3.4 1016
C)6.8 1016
D)6.8 1016
E)2.8 10-15
A)3.4 10-16
B)3.4 1016
C)6.8 1016
D)6.8 1016
E)2.8 10-15

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following experiments most directly supports de Broglie's hypothesis of the wave nature of matter?
A)Black-body radiation
B)The photoelectric effect
C)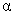 -particle scattering by a metal foil
-particle scattering by a metal foil
D)Electron diffraction by a crystal
E)The emission spectrum of the hydrogen atom
A)Black-body radiation
B)The photoelectric effect
C)
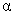 -particle scattering by a metal foil
-particle scattering by a metal foilD)Electron diffraction by a crystal
E)The emission spectrum of the hydrogen atom

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
If an electron is confined to a one-dimensional box 200 pm in length,calculate the zero-point energy.
A)1.51 1018 J
B)6.04 1018 J
C)1.51 1020 J
D)1.21 1019 J
E)1.21 1017 J
A)1.51 1018 J
B)6.04 1018 J
C)1.51 1020 J
D)1.21 1019 J
E)1.21 1017 J

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
If a particle is confined to a one-dimensional box of length 300 pm,for 3 the particle is most likely to be found at
A)0 pm.
B)50, 150, and 250 pm, respectively.
C)17.3 pm.
D)300 pm.
E)100 and 200 pm, respectively.
A)0 pm.
B)50, 150, and 250 pm, respectively.
C)17.3 pm.
D)300 pm.
E)100 and 200 pm, respectively.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
What is the energy of a mole of photons with a frequency of 5.8 1015 sec-1?
A)2.3 10-5J/mol
B)2.3 106J/mol
C)4.9 106J/mol
D)4.9 105J/mol
E)5.8 10-15J/mol
A)2.3 10-5J/mol
B)2.3 106J/mol
C)4.9 106J/mol
D)4.9 105J/mol
E)5.8 10-15J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
In a one-dimensional particle in a box,the zero-point energy corresponds to
A)a node.
B)n = 0.
C)n = 1.
D)A quantum state where the uncertainty principle is not valid.
E)(2 = 0.)
A)a node.
B)n = 0.
C)n = 1.
D)A quantum state where the uncertainty principle is not valid.
E)(2 = 0.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
In a one-dimensional particle in a box,for n = 6,how many wavelengths equal the size of the box?
A)0
B)3
C)1
D)12
E)6
A)0
B)3
C)1
D)12
E)6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
In everyday life,we have no difficulty in measuring both the velocity and position of objects.True or false?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
What is the energy of a mole of photons with a frequency of 9.1 1015 Hz?
A)3.6 10-5J/mol
B)3.6 106J/mol
C)2.8 107J/mol
D)2.8 107J/mol
E)7.0 106J/mol
A)3.6 10-5J/mol
B)3.6 106J/mol
C)2.8 107J/mol
D)2.8 107J/mol
E)7.0 106J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
You are caught in a radar trap and hope to show that the speed measured by the radar gun is in error because of the uncertainty principle.If you assume that the uncertainty in your position is large,say about 10 m,and that the car has a mass of 2150 kg,what is the uncertainty in the velocity?
A)1 1033 ms1
B)1 1019 ms1
C)1 1033 ms1
D)1 1038 ms1
E)1 1038 ms1
A)1 1033 ms1
B)1 1019 ms1
C)1 1033 ms1
D)1 1038 ms1
E)1 1038 ms1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
When a mole of photons has the energy 3.3 107 J,what is its frequency,in Hz?
A)2.6 10-15
B)2.6 1016
C)8.2 1015
D)8.2 1016
E)13.3 10-7
A)2.6 10-15
B)2.6 1016
C)8.2 1015
D)8.2 1016
E)13.3 10-7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Light of wavelength 242 nm ionizes a sodium ion in the gas phase; what is the ionization energy of sodium?
A)8.20 kJmol1
B)988 kJmol1
C)198 kJmol1
D)494 kJmol1
E)247 kJmol1
A)8.20 kJmol1
B)988 kJmol1
C)198 kJmol1
D)494 kJmol1
E)247 kJmol1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
The first line (lowest energy)in the Balmer series appears at 15,233 cm1.The second line appears at
A)109,678 cm1.
B)45,699 cm1.
C)20,565 cm1.
D)30,466 cm1.
E)20,311 cm1.
A)109,678 cm1.
B)45,699 cm1.
C)20,565 cm1.
D)30,466 cm1.
E)20,311 cm1.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
In a one-dimensional particle in a box,for 4,how many nodes are predicted?
A)1
B)3
C)0
D)2
E)4
A)1
B)3
C)0
D)2
E)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Calculate the wavelength of a motorcycle of mass 275 kg traveling at a speed of 125 kmhr1.
A)6.94 1038 m
B)1.93 1038 m
C)1.93 1041 m
D)2.41 1036 m
E)2.08 1029 m
A)6.94 1038 m
B)1.93 1038 m
C)1.93 1041 m
D)2.41 1036 m
E)2.08 1029 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
If a particle is confined to a one-dimensional box of length 300 pm,for 3 the particle has zero probability of being found at
A)100 and 200 pm, respectively.
B)150 pm only.
C)50, 150, and 250 pm, respectively.
D)50 and 250 pm, respectively.
E)75, 125, 175, and 225 pm, respectively.
A)100 and 200 pm, respectively.
B)150 pm only.
C)50, 150, and 250 pm, respectively.
D)50 and 250 pm, respectively.
E)75, 125, 175, and 225 pm, respectively.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
A lawyer who received a speeding ticket argues that because of the Heisenberg uncertainty principle the radar reading is uncertain.The judge,who happens to have a science degree,rules against the lawyer.Which of the following statements is true?
A)The judge is incorrect because the uncertainty in position is large.
B)The judge is correct because the car is so massive that the uncertainty in speed is very small.
C)The judge is correct because the uncertainty in momentum is very large.
D)The judge is incorrect because radar has only wave characteristics.
E)The judge is incorrect because (mv)(x)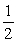 h.
h.
A)The judge is incorrect because the uncertainty in position is large.
B)The judge is correct because the car is so massive that the uncertainty in speed is very small.
C)The judge is correct because the uncertainty in momentum is very large.
D)The judge is incorrect because radar has only wave characteristics.
E)The judge is incorrect because (mv)(x)
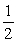 h.
h.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
An electron is confined to a one-dimensional box 200 pm in length,calculate the wavelength of light required to promote the electron from the ground state to the first excited state.
A)329 pm
B)263 pm
C)165 pm
D)439 pm
E)1320 pm
A)329 pm
B)263 pm
C)165 pm
D)439 pm
E)1320 pm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
When a mole of photons possesses an energy of 2.4 107 J,what is its frequency in /sec?
A)1.6 10-15J/mol
B)6.1 10-16J/mol
C)6.1 1016J/mol
D)1.6 105J/mol
E)4.2 1016J/mol
A)1.6 10-15J/mol
B)6.1 10-16J/mol
C)6.1 1016J/mol
D)1.6 105J/mol
E)4.2 1016J/mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
The Bohr radius for an electron in the ground state of a hydrogen atom is 52.9 pm.The Bohr radius for an electron in the n = 2 state of He+ is
A)211.6 pm.
B)105.8 pm.
C)26.5 pm.
D)52.9 pm.
E)13.2 pm.
A)211.6 pm.
B)105.8 pm.
C)26.5 pm.
D)52.9 pm.
E)13.2 pm.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements is incorrect?
A)For a one-dimensional particle in a box, as the mass of the particle becomes larger the separation between neighboring energy levels increases.
B)For a one-dimensional particle in a box, the separation between neighboring energy levels increases as the length of the container increases.
C)For a one-dimensional particle in a box, the energy separation between neighboring energy levels has the value h2/8mL2 when the walls of the box are infinitely far apart.
D)Argon atoms in a cylinder can be treated as though their translational energy were quantized.
E)A billiard ball on a table has a completely negligible zero-point energy.
A)For a one-dimensional particle in a box, as the mass of the particle becomes larger the separation between neighboring energy levels increases.
B)For a one-dimensional particle in a box, the separation between neighboring energy levels increases as the length of the container increases.
C)For a one-dimensional particle in a box, the energy separation between neighboring energy levels has the value h2/8mL2 when the walls of the box are infinitely far apart.
D)Argon atoms in a cylinder can be treated as though their translational energy were quantized.
E)A billiard ball on a table has a completely negligible zero-point energy.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


