Deck 3: Chemical Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/84
Play
Full screen (f)
Deck 3: Chemical Bonds
1
All the following elements exist as diatomic gases at room temperature and atmospheric pressure except
A)H.
B)Ar.
C)N.
D)Cl.
E)O.
A)H.
B)Ar.
C)N.
D)Cl.
E)O.
Ar.
2
If 491 kJmol1 is released in the reaction Na+(g)+ Cl(g) Na+Cl(g),what is the energy change for the reaction Na(g)+ Cl(g) Na+Cl(g)? (Hint: See the discussion in the text and apply Hess's Law.)
346 kJmol1
3
Use the expression for the Coulomb potential energy to calculate the energy for formation of 1 mole of rubidium chloride ion-pairs,that is,the energy change for the following reaction:
Rb+(g)+ Cl(g) Rb+Cl(g)
Use r12 = 330 pm.
Rb+(g)+ Cl(g) Rb+Cl(g)
Use r12 = 330 pm.
421 kJmol1
4
Which of the following has the highest lattice energy?
A)NaCl
B)KI
C)MgO
D)BaO
E)CaO
A)NaCl
B)KI
C)MgO
D)BaO
E)CaO

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
5
For the ground-state ion Pb2+,what type of orbital do the electrons with highest energy reside in?
A)6p
B)5p
C)4f
D)6s
E)5d
A)6p
B)5p
C)4f
D)6s
E)5d

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following metal ions has the ground-state electron configuration [Ar]3d6?
A)Ni3+
B)Fe2+
C)Mn2+
D)Cu+
E)Ca2+
A)Ni3+
B)Fe2+
C)Mn2+
D)Cu+
E)Ca2+

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
7
Predict the electronic configuration in the oxide ion in CaO.
A)[He]2s22p6 or [Ne]
B)[He]2s22p5
C)[He]2s22p63s2
D)[Ne]3s13p3
E)[Ne]3s23p3
A)[He]2s22p6 or [Ne]
B)[He]2s22p5
C)[He]2s22p63s2
D)[Ne]3s13p3
E)[Ne]3s23p3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
8
An element,E,has the electronic configuration [Ne] 3s23p1.Write the formula of its compound with sulfate.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
9
Metals rarely lose electrons in chemical reactions because
A)their electron affinities are too high.
B)their ionic radii become too small.
C)their ionization energies are too small.
D)their size is too small.
E)their ionization energies are too high.
A)their electron affinities are too high.
B)their ionic radii become too small.
C)their ionization energies are too small.
D)their size is too small.
E)their ionization energies are too high.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following has the highest melting point?
A)KF
B)KI
C)RbF
D)KBr
E)KCl
A)KF
B)KI
C)RbF
D)KBr
E)KCl

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
11
How many lone pairs of electrons are found in the Lewis structure of the interhalogen compound ICl3?
A)10
B)4
C)8
D)6
E)7
A)10
B)4
C)8
D)6
E)7

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
12
Use the expression for the Coulomb potential energy to calculate the energy for formation of 1 mole of sodium chloride ion-pairs,that is,the energy change for the following reaction:
Na+(g)+ Cl(g) Na+Cl(g)
Use r12 = 283 pm.
Na+(g)+ Cl(g) Na+Cl(g)
Use r12 = 283 pm.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
13
For the ground-state ion Bi3+,what type of orbital do the electrons with highest energy reside in?
A)5d
B)6s
C)4f
D)5p
E)6p
A)5d
B)6s
C)4f
D)5p
E)6p

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
14
The Madelung constant is different for all crystals.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
15
Because of the octet rule,the gaseous O2 ion is stable.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
16
Write the formula of magnesium phosphide.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following has the lowest lattice energy?
A)KCl
B)LiCl
C)KBr
D)NaCl
E)KI
A)KCl
B)LiCl
C)KBr
D)NaCl
E)KI

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
18
For the ground-state ion I,what type of orbital do the electrons with highest energy reside in?
A)4d
B)6s
C)5p
D)5d
E)5s
A)4d
B)6s
C)5p
D)5d
E)5s

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
19
If 346 kJmol1 is released in the reaction Na(g)+ Cl(g) Na+Cl(g),is the energy change for the reaction Na+Cl(g) NaCl(s)endothermic or exothermic?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
20
For the ground-state ion Sn4+,what type of orbital do the electrons with highest energy reside in?
A)4p
B)5p
C)4f
D)4d
E)5s
A)4p
B)5p
C)4f
D)4d
E)5s

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
21
How many electrons are in the expanded valence in I3?
A)12
B)6
C)10
D)14
E)8
A)12
B)6
C)10
D)14
E)8

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the Lewis structure of xenon difluoride and give the number of lone pairs electrons around the central atom.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
23
How many lone pairs of electrons are found in the Lewis structure of urea,(NH2)2CO?
A)2
B)3
C)6
D)4
E)8
A)2
B)3
C)6
D)4
E)8

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
24
In the "best" Lewis structure of XeO4,there are two double bonds and the formal charge on Xe is zero.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the Lewis structure of the formate ion and indicate whether resonance forms are possible.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
26
How many lone pairs of electrons are found in the Lewis structure of hydrazine,H2NNH2?
A)8
B)4
C)1
D)0
E)2
A)8
B)4
C)1
D)0
E)2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the N-O bond lengths in NO2,given the N-O and N=O bond lengths of 140 and 120 pm,respectively.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
28
For dinitrogen monoxide,the arrangement of the atoms is N-N-O.In the Lewis structure with a single bond between NN and a triple bond between NO,the formal charges on N,N,and O,respectively,are
A)(1, +1, 0.)
B)0, 0, 0.
C)0, +1, 1.
D)0, 1, +1.
E)(2, +1, +1.)
A)(1, +1, 0.)
B)0, 0, 0.
C)0, +1, 1.
D)0, 1, +1.
E)(2, +1, +1.)

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
29
Why are the N-O bond lengths in NO3 the same?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
30
Draw the "best" Lewis structures of hydrogen azide,HN1N2N3,and the azide ion,N1N2N3 .The subscripts are used for identification.For each,match the following bond lengths to the correct N-N bond.The bond lengths can be used more than once.
N-N bond
Bond length,pm
hydrogen azide
N1-N2
113
N2-N3
116
azide ion
N1-N2
124
N2-N3
N-N bond
Bond length,pm
hydrogen azide
N1-N2
113
N2-N3
116
azide ion
N1-N2
124
N2-N3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
31
How many electrons are in the expanded valence in XeO4?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following equilibrium:
S2O42(aq)↔ 2SO2(aq)
K ~ 109
Write a Lewis structure for each species.
S2O42(aq)↔ 2SO2(aq)
K ~ 109
Write a Lewis structure for each species.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
33
How many electrons are in the expanded valence in H2SO4?
A)12
B)14
C)8
D)6
E)10
A)12
B)14
C)8
D)6
E)10

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
34
Write three Lewis structures for the cyanate ion,NCO,where the arrangement of atoms is N-C-O.In the most plausible structure,
A)there is a triple bond between N and C.
B)there are two double bonds.
C)there is a triple bond between C and O.
D)the formal charge on O is +1.
E)the formal charge on N is 1.
A)there is a triple bond between N and C.
B)there are two double bonds.
C)there is a triple bond between C and O.
D)the formal charge on O is +1.
E)the formal charge on N is 1.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
35
In the most plausible Lewis structure of XeOF2,there are
A)2 single bonds, 1 double bond, and 1 lone pair of electrons around Xe.
B)3 single bonds and 1 lone pair of electrons around Xe.
C)2 single bonds, 1 double bond, and 3 lone pairs of electrons around Xe.
D)2 single bonds, 1 double bond, and 2 lone pairs of electrons around Xe.
E)3 single bonds and 2 lone pairs of electrons around Xe.
A)2 single bonds, 1 double bond, and 1 lone pair of electrons around Xe.
B)3 single bonds and 1 lone pair of electrons around Xe.
C)2 single bonds, 1 double bond, and 3 lone pairs of electrons around Xe.
D)2 single bonds, 1 double bond, and 2 lone pairs of electrons around Xe.
E)3 single bonds and 2 lone pairs of electrons around Xe.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following species are radicals?
A)CO2
B)HNO3
C)NO2
D)NO3
A)CO2
B)HNO3
C)NO2
D)NO3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
37
For dinitrogen monoxide,the arrangement of the atoms is N-N-O.In the Lewis structure with a double bond between NN and NO,the formal charges on N,N,and O,respectively,are
A)0, 1, +1.
B)(1, +1, 0.)
C)0, +1, 1.
D)0, 0, 0.
E)(2, +1, +1.)
A)0, 1, +1.
B)(1, +1, 0.)
C)0, +1, 1.
D)0, 0, 0.
E)(2, +1, +1.)

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following species are radicals?
A)CH2O
B)HCN
C)HclO
D)ClONO2
E)ClO
A)CH2O
B)HCN
C)HclO
D)ClONO2
E)ClO

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following do not have resonance structures?
A)CH3CONH
B)CH2COCH3
C)H2CO
D)All have resonance structures.
A)CH3CONH
B)CH2COCH3
C)H2CO
D)All have resonance structures.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
40
How many electrons are in the expanded valence in XeOF2?
A)14
B)12
C)8
D)10
E)6
A)14
B)12
C)8
D)10
E)6

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
41
Estimate the CO bond length in acetone,CH3COCH3.Given: covalent radii (pm)of C-,77; C=,67; O-,74; O=,60; H,37.
A)75.5 pm
B)127 pm
C)63.5 pm
D)151 pm
E)137 pm
A)75.5 pm
B)127 pm
C)63.5 pm
D)151 pm
E)137 pm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following compounds contains the weakest bonds to hydrogen?
A)CH4
B)H2O
C)SiH4
D)HF
E)H2S
A)CH4
B)H2O
C)SiH4
D)HF
E)H2S

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
43
Use the bond enthalpies given to estimate the heat released when 2-methyl-1-propene,(CH3)2C=CH2,reacts with HBr to give (CH3)2CBrCH3.Bond enthalpies (kJmol1): C-H,412; C-C,348; C=C,612; C-Br,276; H-Br,366.
A)58 kJmol1
B)507 kJmol1
C)317 kJmol1
D)288 kJmol1
E)181 kJmol1
A)58 kJmol1
B)507 kJmol1
C)317 kJmol1
D)288 kJmol1
E)181 kJmol1

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
44
If the following all crystallize in the same type of structure,which has the highest lattice energy?
A)LiCl
B)KF
C)KBr
D)KCl
E)LiF
A)LiCl
B)KF
C)KBr
D)KCl
E)LiF

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
45
Use the bond enthalpies given to estimate the heat released when ethene,CH2=CH2,reacts with hydrogen to give CH3CH3.Bond enthalpies (kJmol1): C-H,412; C-C,348; C=C,612; C-Br,276; H-H,436.
A)124 kJmol1
B)342 kJmol1
C)288 kJmol1
D)148 kJmol1
E)560 kJmol1
A)124 kJmol1
B)342 kJmol1
C)288 kJmol1
D)148 kJmol1
E)560 kJmol1

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following compounds is the least stable?
A)CH4
B)SnH4
C)SiH4
D)GeH4
E)PbH4
A)CH4
B)SnH4
C)SiH4
D)GeH4
E)PbH4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
47
Write all possible Lewis structures of sulfur dioxide.Which structure is most feasible?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements is true?
A)The electronegativity of an atom is defined as electron affinity of the atom.
B)The electronegativity of an atom depends only on the value of the ionization energy of the atom.
C)Atoms with high ionization energies and high electron affinities have low electronegativities.
D)Atoms with low ionization energies and low electron affinities have low electronegativities.
E)Atoms with low ionization energies and low electron affinities have high electronegativities.
A)The electronegativity of an atom is defined as electron affinity of the atom.
B)The electronegativity of an atom depends only on the value of the ionization energy of the atom.
C)Atoms with high ionization energies and high electron affinities have low electronegativities.
D)Atoms with low ionization energies and low electron affinities have low electronegativities.
E)Atoms with low ionization energies and low electron affinities have high electronegativities.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
49
Use the bond enthalpies given to estimate the heat released when ethene,CH2=CH2,reacts with HBr to give CH3CH2Br.Bond enthalpies (kJmol1): C-H,412; C-C,348; C=C,612; C-Br,276; Br-Br,193; H-Br,366.
A)1036 kJmol1
B)200 kJmol1
C)470 kJmol1
D)424 kJmol1
E)58 kJmol1
A)1036 kJmol1
B)200 kJmol1
C)470 kJmol1
D)424 kJmol1
E)58 kJmol1

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
50
If the following all crystallize in the same type of structure,which has the lowest lattice energy?
A)CaO
B)BaS
C)SrO
D)SrS
E)BaO
A)CaO
B)BaS
C)SrO
D)SrS
E)BaO

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
51
If the following all crystallize in the same type of structure,which has the highest lattice energy?
A)NaCl
B)NaF
C)KF
D)NaBr
E)NaI
A)NaCl
B)NaF
C)KF
D)NaBr
E)NaI

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the compounds below has bonds with the most covalent character?
A)NaCl
B)LiCl
C)CaCl2
D)BeCl2
E)MgCl2
A)NaCl
B)LiCl
C)CaCl2
D)BeCl2
E)MgCl2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
53
Estimate the CN bond length in urea,NH2CONH2.Given: covalent radii (pm)of C-,77; C=,67; N-,75; N=,60; O-,74; O=,60; H,37.
A)71 pm
B)127 pm
C)76 pm
D)152 pm
E)142 pm
A)71 pm
B)127 pm
C)76 pm
D)152 pm
E)142 pm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the compounds below has bonds with the most covalent character?
A)CaO
B)Li2O
C)MgO
D)MgS
E)CaS
A)CaO
B)Li2O
C)MgO
D)MgS
E)CaS

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following species has bonds with the most ionic character?
A)SiO2
B)PCl3
C)P4O10
D)CO2
E)NO2
A)SiO2
B)PCl3
C)P4O10
D)CO2
E)NO2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following statements is true?
A)Atoms with high ionization energies and high electron affinities are highly electronegative.
B)Atoms with high ionization energies and high electron affinities have low electronegativities.
C)The electronegativity of an atom depends only on the value of the ionization energy of the atom.
D)Atoms with low ionization energies and low electron affinities have high electronegativities.
E)The electronegativity of an atom is defined as half the electron affinity of the atom.
A)Atoms with high ionization energies and high electron affinities are highly electronegative.
B)Atoms with high ionization energies and high electron affinities have low electronegativities.
C)The electronegativity of an atom depends only on the value of the ionization energy of the atom.
D)Atoms with low ionization energies and low electron affinities have high electronegativities.
E)The electronegativity of an atom is defined as half the electron affinity of the atom.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds contains the strongest bonds to hydrogen?
A)SiH4
B)CH4
C)HF
D)H2S
E)H2O
A)SiH4
B)CH4
C)HF
D)H2S
E)H2O

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the compounds below has bonds with the least covalent character?
A)AgI
B)AgCl
C)AgF
D)AlCl3
E)BeCl2
A)AgI
B)AgCl
C)AgF
D)AlCl3
E)BeCl2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
59
Use the bond enthalpies given to estimate the heat released when 1-bromobutene,CH3CH2CH=CH2,reacts with bromine to give CH3CH2CHBrCH2Br.Bond enthalpies (kJmol1): C-H,412; C-C,348; C=C,612; C-Br,276; Br-Br,193.
A)181 kJmol1
B)317 kJmol1
C)288 kJmol1
D)95 kJmol1
E)507 kJmol1
A)181 kJmol1
B)317 kJmol1
C)288 kJmol1
D)95 kJmol1
E)507 kJmol1

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following species has bonds with the most ionic character?
A)CO2
B)NO2
C)SnO2
D)P4O10
E)PCl3
A)CO2
B)NO2
C)SnO2
D)P4O10
E)PCl3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
61
If the following all crystallize in the same type of structure,which has the lowest lattice energy?
A)LiCl
B)NaI
C)NaCl
D)KCl
E)KI
A)LiCl
B)NaI
C)NaCl
D)KCl
E)KI

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
62
Of the following molecules,which has the strongest bonds?
A)H2O
B)H2Se
C)H2Te
D)H2S
A)H2O
B)H2Se
C)H2Te
D)H2S

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
63
The best Lewis structures of SO2 and O3 include expanded valence structures such as O=S=O and O=O=O.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
64
An element E has the electronic configuration 1s22s22p4.What is the formula of its compound with lithium?
A)LiE2
B)LiE
C)Li2E
D)Li4E
A)LiE2
B)LiE
C)Li2E
D)Li4E

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
65
Sulfur is more electronegative than oxygen.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following has resonance structures?
A)XeOF2
B)N2H4
C)CH3CONH
D)H2CO
A)XeOF2
B)N2H4
C)CH3CONH
D)H2CO

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
67
How many valence electrons are present in W4+?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
68
How many resonance structures can be drawn for N2O?
A)0
B)3
C)2
D)1
A)0
B)3
C)2
D)1

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
69
The electronegativity of an element can be expressed as ½(I + Ea)where I is the ionization energy and Ea is the electron affinity.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
70
White phosphorus is composed of tetrahedral molecules of P4 in which every P atom is connected to three other P atoms.In the Lewis structure of P4,there are
A)3 bonding pairs and 4 lone pairs of electrons.
B)6 bonding pairs and 2 lone pairs of electrons.
C)5 bonding pairs and 4 lone pairs of electrons.
D)6 bonding pairs and no lone pairs of electrons.
E)6 bonding pairs and 4 lone pairs of electrons.
A)3 bonding pairs and 4 lone pairs of electrons.
B)6 bonding pairs and 2 lone pairs of electrons.
C)5 bonding pairs and 4 lone pairs of electrons.
D)6 bonding pairs and no lone pairs of electrons.
E)6 bonding pairs and 4 lone pairs of electrons.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
71
White phosphorus is composed of tetrahedral molecules of P4 in which each P atom is bonded to three others.In this molecule the formal charge on each P atom is ___.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
72
Match each of the following compounds with its lattice energy.
KI,LiF,MgF2,LiI 2961,1046,759,645 kJ/mol
KI,LiF,MgF2,LiI 2961,1046,759,645 kJ/mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
73
How many double bonds are present in the "best" resonance structure of the phosphate ion?
A)2
B)3
C)1
D)0
A)2
B)3
C)1
D)0

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
74
What is the electronic configuration of Ag?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is a radical?
A)BrO
B)CH3+
C)CH3
D)BF4
A)BrO
B)CH3+
C)CH3
D)BF4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
76
If dinitrogen oxide has a dipole moment,what is the arrangement of atoms?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
77
There are three resonance structures of the sulfate ion.A resonance structure can be written where the formal charge on sulfur is 0.True or false?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
78
What is the formal charge on the Xe atom in XeF4?
A)0
B)(4)
C)+2
D)+4
A)0
B)(4)
C)+2
D)+4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
79
How many lone pairs of electrons are there in the Lewis structure of Al2Cl6?
A)24
B)12
C)4
D)16
A)24
B)12
C)4
D)16

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
80
What is wrong with the following Lewis structure? 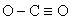
A)The valence electron count
B)The positioning of the carbon atom
C)The distribution of valence electrons
D)The charge on the carbon atom
E)The dipole of the molecule
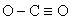
A)The valence electron count
B)The positioning of the carbon atom
C)The distribution of valence electrons
D)The charge on the carbon atom
E)The dipole of the molecule

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck


