Deck 5: Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/191
Play
Full screen (f)
Deck 5: Equilibrium
1
In a pressure cooker,the boiling point of water is less than 100 C.
False
2
Estimate the enthalpy of vaporization of CCl4 given that at 25 C and 58 C its vapor pressure is 107 and 405 Torr, respectively.Assume that the enthalpy of vaporization is independent of the temperature.
A) 486 J.mol-1
B) 48.6 kJ.mol-1
C) 142 kJ.mol-1
D) 3.98 kJ.mol-1
E) 33.1 kJ.mol-1
A) 486 J.mol-1
B) 48.6 kJ.mol-1
C) 142 kJ.mol-1
D) 3.98 kJ.mol-1
E) 33.1 kJ.mol-1
33.1 kJ.mol-1
3
The phase diagram for a pure substance is shown below. 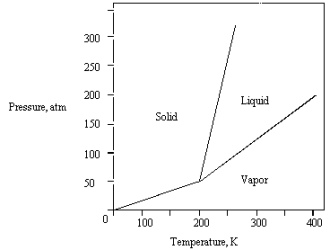 What is the lowest temperature at which liquid can exist?
What is the lowest temperature at which liquid can exist?
A) 400 K
B) 0 K
C) 200 K
D) 150 K
E) 250 K
 What is the lowest temperature at which liquid can exist?
What is the lowest temperature at which liquid can exist?A) 400 K
B) 0 K
C) 200 K
D) 150 K
E) 250 K
C
4
A plot of ln(vapor pressure) versus 1/T for methanol gives a straight line with an intercept of 13.6.The entropy of vaporization of methanol is
A) 611 J.mol-1.K-1.
B) 113 J.mol-1.K-1.
C) 1640 J.mol-1.K-1.
D) Not enough information given to calculate.
E) 13.6 J.mol-1.K-1.
A) 611 J.mol-1.K-1.
B) 113 J.mol-1.K-1.
C) 1640 J.mol-1.K-1.
D) Not enough information given to calculate.
E) 13.6 J.mol-1.K-1.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
5
The phase diagram for a pure compound is given below .All of the following could have a similar phase diagram except 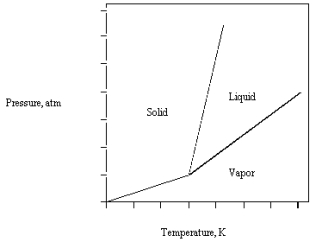
A) methanol
B) carbon dioxide
C) benzene
D) carbon tetrachloride
E) water

A) methanol
B) carbon dioxide
C) benzene
D) carbon tetrachloride
E) water

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
6
The vapor pressure of water above 40 mL of water in a 100 mL container is 23.8 Torr at 25 C .What is the vapor pressure of the water if the volume of the container is changed to 50 mL?
A) About 25 Torr
B) About 20 Torr
C) 23.8 Torr
D) 47.6 Torr
E) 11.9 Torr
A) About 25 Torr
B) About 20 Torr
C) 23.8 Torr
D) 47.6 Torr
E) 11.9 Torr

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
7
Estimate the enthalpy of vaporization of water given that at 25 C and 35F C its vapor pressure is 23.8 and 42 Torr, respectively.Assume that the enthalpy of vaporization is independent of the temperature.
A) 415 J.mol-1
B) 221 kJ.mol-1
C) 41.5 kJ.mol-1
D) 5.21 kJ.mol-1
E) 43.4 kJ.mol-1
A) 415 J.mol-1
B) 221 kJ.mol-1
C) 41.5 kJ.mol-1
D) 5.21 kJ.mol-1
E) 43.4 kJ.mol-1

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
8
In a closed vessel containing water the pressure is 18 Torr .If more water is added to the vessel,The pressure
A) remains the same.
B) becomes larger.
C) becomes smaller.
A) remains the same.
B) becomes larger.
C) becomes smaller.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
9
The vapor pressure of benzene at 25 C is 94.6 Torr and its enthalpy of vaporization is 30.8 kJ.mol-1 . Estimate the normal boiling point of benzene.Assume the enthalpy of vaporization is independent of temperature.
A) 640 K
B) 358 K
C) 470 K
D) 624 K
E) Not enough information given.
A) 640 K
B) 358 K
C) 470 K
D) 624 K
E) Not enough information given.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
10
The vapor pressure of methanol at 25 C is 123 Torr and its enthalpy of vaporization is 35.3 kJ.mol-1 . Estimate the normal boiling point of methanol.Assume the enthalpy of vaporization is independent of temperature.
A) 450 K
B) 342 K
C) 315 K
D) 373 K
E) Not enough information given.
A) 450 K
B) 342 K
C) 315 K
D) 373 K
E) Not enough information given.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
11
A plot of ln(vapor pressure) versus 1/T for benzene gives a straight line with slope -3.70 * 103 K.The enthalpy of vaporization of benzene is
A) 2.25 kJ.mol-1.
B) Not enough information is given to permit the calculation.
C) 3.70 kJ.mol-1.
D) 30.8 kJ.mol-1.
E) 445 J.mol-1.
A) 2.25 kJ.mol-1.
B) Not enough information is given to permit the calculation.
C) 3.70 kJ.mol-1.
D) 30.8 kJ.mol-1.
E) 445 J.mol-1.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
12
Why is the vapor pressure of cis-dibromoethene lower than the vapor pressure of trans-dibromoethene?
A) Ion-dipole forces
B) London forces
C) Hydrogen bonding
D) Ion-ion forces
E) Dipole-dipole forces
A) Ion-dipole forces
B) London forces
C) Hydrogen bonding
D) Ion-ion forces
E) Dipole-dipole forces

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
13
A plot of ln(vapor pressure) versus 1/T for methanol gives a straight line with an intercept of 13.6.The enthalpy of vaporization of methanol is
A) 113 kJ.mol-1.
B) 13.6 kJ.mol-1.
C) 611 kJ.mol-1.
D) 1.64 kJ.mol-1.
E) Not enough information given to calculate.
A) 113 kJ.mol-1.
B) 13.6 kJ.mol-1.
C) 611 kJ.mol-1.
D) 1.64 kJ.mol-1.
E) Not enough information given to calculate.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
14
Which liquid would you expect to be the more volatile,CH3CHO or CH3OCH3?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
15
What is the vapor pressure of carbon disulfide at its normal boiling point?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following has the highest vapor pressure?
A) H2O
B) CH3CH2OH
C) CH3CH2OCH2CH3
D) CH3OH
E) CH3CH2COOH
A) H2O
B) CH3CH2OH
C) CH3CH2OCH2CH3
D) CH3OH
E) CH3CH2COOH

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
17
The vapor pressure of water at 37 C is 47.1 Torr and its enthalpy of vaporization is 44.0 kJ.mol-1 .Estimate the vapor pressure of water at 87 C.Assume that the enthalpy of vaporization of water is independent of temperature.
A) 112 Torr
B) 256 Torr
C) 713 Torr
D) 52 Torr
E) 504 Torr
A) 112 Torr
B) 256 Torr
C) 713 Torr
D) 52 Torr
E) 504 Torr

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following liquids freeze at a lower temperature when pressure is applied?
A) Water
B) Acetic acid
C) Benzene
D) Methanol
E) Carbon tetrachloride
A) Water
B) Acetic acid
C) Benzene
D) Methanol
E) Carbon tetrachloride

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
19
The phase diagram for a pure compound is shown below .The triple point occurs at 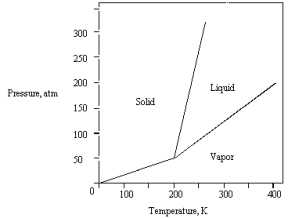
A) 50 atm and 200 K.
B) 0 atm and 200 K.
C) greater than 50 atm and greater than 200 K.
D) 320 atm and 250 K.
E) 200 atm and 400 K.

A) 50 atm and 200 K.
B) 0 atm and 200 K.
C) greater than 50 atm and greater than 200 K.
D) 320 atm and 250 K.
E) 200 atm and 400 K.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
20
the boiling point of water is higher in a pressure cooker than the normal boiling point?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
21
the triple point for water,4.6 Torr and 0.01 C,varies depending on conditions?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
22
The phase diagram for a pure substance is given below. 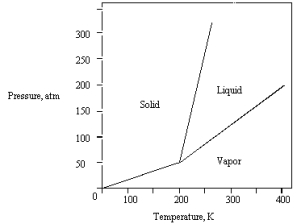 The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
A) The liquid in the container freezes.
B) The solid in the container sublimes.
C) The solid in the container melts.
D) The vapor in the container escapes.
E) The liquid in the container vaporizes.
 The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.
The substance is stored in a container at 150 atm at 25 C.Describe what happens if the container is opened at 25 C.A) The liquid in the container freezes.
B) The solid in the container sublimes.
C) The solid in the container melts.
D) The vapor in the container escapes.
E) The liquid in the container vaporizes.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
23
For a one-component system, at the triple point
A) f = 3.
B) f = 2.
C) p = 1.
D) f = 0.
E) f = 1.
A) f = 3.
B) f = 2.
C) p = 1.
D) f = 0.
E) f = 1.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
24
The phase diagram for CO2 is given below .
The triple point is at 5.1 atm and 217 K.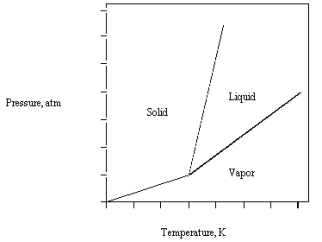
What happens if carbon dioxide at 50C and 25 atm is suddenly brought to 1 atm?
A) The liquid and solid are in equilibrium.
B) The solid melts.
C) The solid and vapor are in equilibrium.
D) The solid vaporizes.
E) The solid remains stable.
The triple point is at 5.1 atm and 217 K.

What happens if carbon dioxide at 50C and 25 atm is suddenly brought to 1 atm?
A) The liquid and solid are in equilibrium.
B) The solid melts.
C) The solid and vapor are in equilibrium.
D) The solid vaporizes.
E) The solid remains stable.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
25
The phase diagram for a pure substance is shown below. 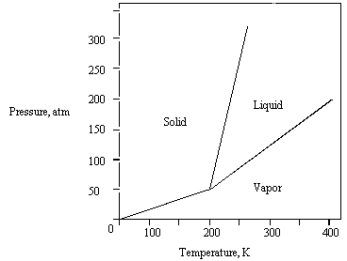 At 100 atm and 250 K,
At 100 atm and 250 K,
The substance exists as
A) both vapor and liquid in equilibrium.
B) liquid.
C) both vapor and solid in equilibrium.
D) vapor.
E) solid.
 At 100 atm and 250 K,
At 100 atm and 250 K,The substance exists as
A) both vapor and liquid in equilibrium.
B) liquid.
C) both vapor and solid in equilibrium.
D) vapor.
E) solid.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
26
The phase diagram for a pure substance is given below .What is the critical temperature? 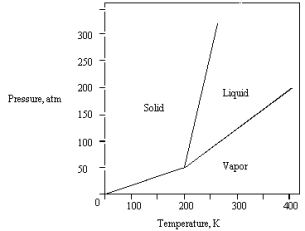
A) 0 K
B) 250 K
C) 300 K
D) 400 K
E) 200 K

A) 0 K
B) 250 K
C) 300 K
D) 400 K
E) 200 K

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
27
What is the highest temperature that the substance shown in the following phase diagram can exist as a liquid? 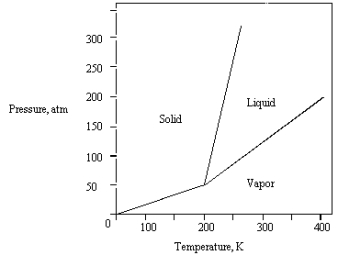
A) any temperature above 200 K
B) 200 K
C) 250 K
D) 350 K
E) 400 K

A) any temperature above 200 K
B) 200 K
C) 250 K
D) 350 K
E) 400 K

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
28
When three phases are in mutual equilibrium,such as at the triple point of water,the degrees of freedom equal 0.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
29
The phase diagram for a pure substance is given below .
The solid sublimes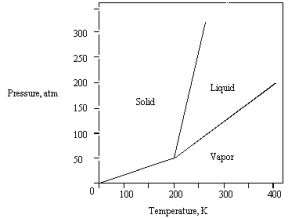
A) at 400 K and 200 atm.
B) at 200 K and 100 atm.
C) at 300 K and 100 atm.
D) at 300 K and 75 atm.
E) if warmed at any pressure below 50 atm.
The solid sublimes

A) at 400 K and 200 atm.
B) at 200 K and 100 atm.
C) at 300 K and 100 atm.
D) at 300 K and 75 atm.
E) if warmed at any pressure below 50 atm.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the phase diagrams for water and carbon dioxide given in the text on page 315.Explain the following observations: A thin wire with weights attached is draped over a block of "dry ice," a second wire with weights is draped over a block of ice.The wire cuts through the ice but not through the "dry ice."

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
31
The phase diagram for sulfur is given below. 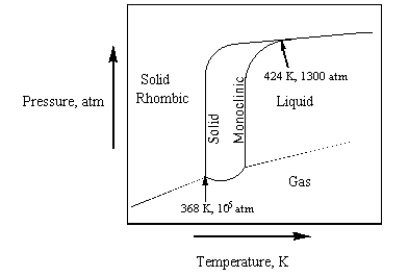 The number of phases that sulfur can exist in and the number of triple points,Respectively,Are
The number of phases that sulfur can exist in and the number of triple points,Respectively,Are
A) 3 and 2.
B) 3 and 3.
C) 4 and 2.
D) 3 and 1.
E) 4 and 3.
 The number of phases that sulfur can exist in and the number of triple points,Respectively,Are
The number of phases that sulfur can exist in and the number of triple points,Respectively,AreA) 3 and 2.
B) 3 and 3.
C) 4 and 2.
D) 3 and 1.
E) 4 and 3.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the phase diagram for sulfur in the text on page 316.Monoclinic sulfur is denser than rhombic sulfur.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
33
The phase diagram for a pure substance is shown below .What is the critical pressure? 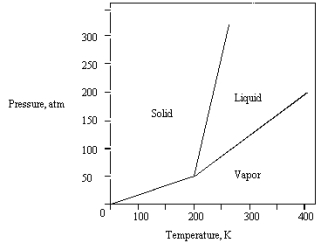
A) 50 atm
B) 150 atm
C) 325 atm
D) Any pressure above 325 atm
E) 200 atm

A) 50 atm
B) 150 atm
C) 325 atm
D) Any pressure above 325 atm
E) 200 atm

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
34
The phase diagram for sulfur is given below. 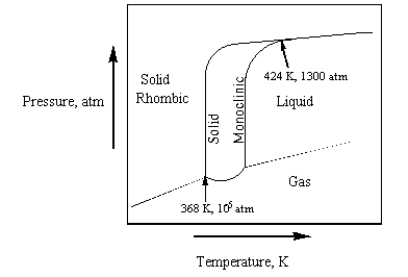 Which of the following is true?
Which of the following is true?
A) Sulfur has 2 triple points.
B) Monoclinic sulfur does not sublime.
C) Sulfur has 0 triple points.
D) Sulfur has 3 triple points.
E) Rhombic sulfur cannot be directly converted to liquid.
 Which of the following is true?
Which of the following is true?A) Sulfur has 2 triple points.
B) Monoclinic sulfur does not sublime.
C) Sulfur has 0 triple points.
D) Sulfur has 3 triple points.
E) Rhombic sulfur cannot be directly converted to liquid.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
35
The phase diagram for CO2 is given below .The triple point is at 5.1 atm and 217 K.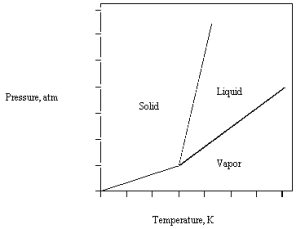
What happens if CO2(l)At 30 atm and 450 K is released into a room at 1 atm and 298 K?
A) The liquid vaporizes.
B) The liquid remains stable.
C) The liquid and vapor are in equilibrium.
D) The liquid and solid are in equilibrium.
E) The liquid freezes.

What happens if CO2(l)At 30 atm and 450 K is released into a room at 1 atm and 298 K?
A) The liquid vaporizes.
B) The liquid remains stable.
C) The liquid and vapor are in equilibrium.
D) The liquid and solid are in equilibrium.
E) The liquid freezes.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the phase diagrams for water and carbon dioxide given in the text on page 315.Explain the significance of the slopes of the solid-liquid lines.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
37
A gas is a form of matter that can be liquefied at any temperature by pressure alone.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
38
The phase diagram for a pure substance is given below. 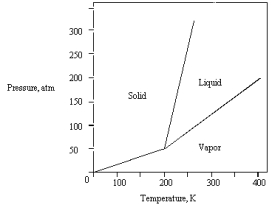 What pressure must be applied to liquefy a sample at 425 K?
What pressure must be applied to liquefy a sample at 425 K?
A) 350 atm
B) The sample cannot be liquefied at 425 K.
C) 150 atm
D) 50 atm
E) 250 atm
 What pressure must be applied to liquefy a sample at 425 K?
What pressure must be applied to liquefy a sample at 425 K?A) 350 atm
B) The sample cannot be liquefied at 425 K.
C) 150 atm
D) 50 atm
E) 250 atm

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
39
The phase diagram for sulfur is given below. 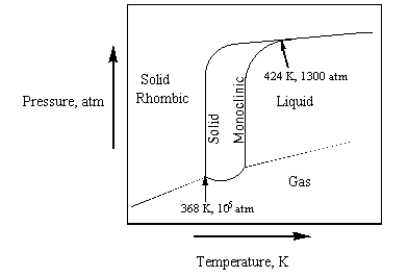 At 424 K and 1300 atm,
At 424 K and 1300 atm,
A) rhombic sulfur, monoclinic sulfur, sulfur liquid, and sulfur gas exist in equilibrium.
B) only rhombic sulfur and sulfur gas exist in equilibrium.
C) rhombic sulfur, monoclinic sulfur, and liquid sulfur exist in equilibrium.
D) only rhombic sulfur is present.
E) only monoclinic sulfur is present.
 At 424 K and 1300 atm,
At 424 K and 1300 atm,A) rhombic sulfur, monoclinic sulfur, sulfur liquid, and sulfur gas exist in equilibrium.
B) only rhombic sulfur and sulfur gas exist in equilibrium.
C) rhombic sulfur, monoclinic sulfur, and liquid sulfur exist in equilibrium.
D) only rhombic sulfur is present.
E) only monoclinic sulfur is present.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
40
The phase diagram for carbon dioxide is given below. 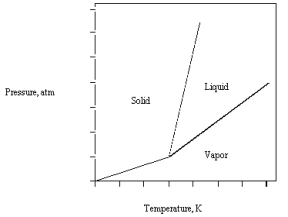 If the triple point is at 5.1 atm and 56C,
If the triple point is at 5.1 atm and 56C,
At 1 atm and room temperature
A) solid carbon dioxide is the stable phase.
B) liquid carbon dioxide is the stable phase.
C) gaseous carbon dioxide condenses.
D) gaseous carbon dioxide is the stable phase.
E) solid carbon dioxide melts.
 If the triple point is at 5.1 atm and 56C,
If the triple point is at 5.1 atm and 56C,At 1 atm and room temperature
A) solid carbon dioxide is the stable phase.
B) liquid carbon dioxide is the stable phase.
C) gaseous carbon dioxide condenses.
D) gaseous carbon dioxide is the stable phase.
E) solid carbon dioxide melts.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
41
For AlF3,the lattice enthalpy is 5220 kJ.mol-1 and the enthalpy of solution is -27 kJ.mol-1 at 25 C.Calculate the enthalpy of hydration of AlF3 at this temperature.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
42
The enthalpy of hydration of AgBr is -819 kJ.mol-1 at 25 C .Given that the hydration enthalpy of Br- is -309 kJ.mol-1,Calculate the enthalpy of hydration of Ag+ ions.
A) -410 kJ.mol-1
B) -819 kJ.mol-1
C) -1128 kJ.mol-1
D) -309 kJ.mol-1
E) -510 kJ?mol?1
A) -410 kJ.mol-1
B) -819 kJ.mol-1
C) -1128 kJ.mol-1
D) -309 kJ.mol-1
E) -510 kJ?mol?1

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements is true regarding solubility? Assume that the solute does not affect the solvent and we are dealing with dilute solutions.
A) We can expect substances with positive enthalpies of solution always to be soluble.
B) We can expect substances with positive enthalpies of solution always to be soluble only if the entropy of solution is negative.
C) All the other statements are false because the solute always affects the solvent resulting in a negative entropy of solution.
D) We can expect substances with negative enthalpies of solution always to be soluble.
E) We can expect substances with positive enthalpies of solution always to be soluble only if the entropy of solution is positive.
A) We can expect substances with positive enthalpies of solution always to be soluble.
B) We can expect substances with positive enthalpies of solution always to be soluble only if the entropy of solution is negative.
C) All the other statements are false because the solute always affects the solvent resulting in a negative entropy of solution.
D) We can expect substances with negative enthalpies of solution always to be soluble.
E) We can expect substances with positive enthalpies of solution always to be soluble only if the entropy of solution is positive.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
44
Aqueous ammonia (28%)is 15.0 M and has a density of 0.90 g.mL-1.Calculate the molality of ammonia in this solution.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
45
What is the molality of CrCl3 in a solution prepared by dissolving 75.2 g of chromium(III) chloride hexahydrate in 250.0 g of water.
A) 0.282 m
B) 1.13 m
C) 7.60 m
D) 5.64 m
E) 1.90 m
A) 0.282 m
B) 1.13 m
C) 7.60 m
D) 5.64 m
E) 1.90 m

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
46
What is the molality of carbon tetrachloride in a solution of toluene,given that the mole fraction is 0.200?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
47
Benzene would likely dissolve which of the following substances?
A) Cl2CCCl2
B) NaCl
C) Na2CO3
D) Ca(HCO3)2
E) C6H12O6
A) Cl2CCCl2
B) NaCl
C) Na2CO3
D) Ca(HCO3)2
E) C6H12O6

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the concentration of argon in lake water at 20?C .The partial pressure of argon is 0.0090 atm and Henrys constant is 0.0015 mol?L?1?atm?1.
A) 1.5 ? 10?3 M
B) 9.0 ? 10?3 M
C) 1.4 ? 10?5 M
D) 6.0 M
E) 0.17 M
A) 1.5 ? 10?3 M
B) 9.0 ? 10?3 M
C) 1.4 ? 10?5 M
D) 6.0 M
E) 0.17 M

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
49
For CaCl2,the enthalpies of hydration and solution are -2337 and -81 kJ.mol-1,respectively,at 25 C.Calculate the lattice enthalpy of calcium chloride.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is likely to have the largest exothermic hydration enthalpy?
A) Sr2+
B) Na+
C) Mg2+
D) Ca2+
E) Li+
A) Sr2+
B) Na+
C) Mg2+
D) Ca2+
E) Li+

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
51
If you wanted to study solutions of different concentrations at different temperatures,would you use molarity or molality as your measure of concentration? Why?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
52
Calculate the molality of ethanol in a solution of water,given that the mole fraction of ethanol is 0.300.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
53
For AgI, the lattice enthalpy is larger than the absolute value of the enthalpy of hydration.This means that for AgI
A) Hsol is positive.
B) the solubility increases when the temperature decreases.
C) Hhyd is positive.
D) Hsol is negative.
E) HL is negative.
A) Hsol is positive.
B) the solubility increases when the temperature decreases.
C) Hhyd is positive.
D) Hsol is negative.
E) HL is negative.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the number of moles of oxygen that will dissolve in 45 L of water at 20 C if the partial pressure of oxygen is 0.21 atm .Henry's constant for oxygen is 0.0013 mol.L-1.atm-1.
A) 0.0062 M
B) 0.0013 M
C) 0.012 M
D) 0.00027 M
E) 0.28
A) 0.0062 M
B) 0.0013 M
C) 0.012 M
D) 0.00027 M
E) 0.28

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
55
For CaCl2, the absolute value of the enthalpy of hydration is larger than the lattice enthalpy.This means that for CaCl2
A) the lattice enthalpy is negative.
B) the enthalpy of solution is endothermic.
C) the solubility increases when the temperature increases.
D) the enthalpy of hydration is positive.
E) the enthalpy of solution is exothermic.
A) the lattice enthalpy is negative.
B) the enthalpy of solution is endothermic.
C) the solubility increases when the temperature increases.
D) the enthalpy of hydration is positive.
E) the enthalpy of solution is exothermic.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
56
The condition called the bends results from the rapid release of nitrogen gas in the bloodstream when the diver returns to the surface.Would argon gas or hydrogen gas be suitable replacements for nitrogen gas in "compressed air?" Explain.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the molality of perchloric acid in 9.2 M HClO4(aq).The density of this solution is 1.54 g/mL.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is likely to have the smallest exothermic hydration enthalpy?
A) F
B) I
C) NO3
D) Cl
E) Br
A) F
B) I
C) NO3
D) Cl
E) Br

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the vapor pressure at25 C of a mixture of benzene and toluene in which the mole fraction of benzene is 0.650 .The vapor pressure at 25 C of benzene is 94.6 Torr and that of toluene is 29.1 Torr.
A) 84.4 Torr
B) 124 Torr
C) 51.3 Torr
D) 71.7 Torr
E) 61.5 Torr
A) 84.4 Torr
B) 124 Torr
C) 51.3 Torr
D) 71.7 Torr
E) 61.5 Torr

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
60
In a solution of NaClO4 prepared by dissolving 125 g of sodium perchlorate monohydrate in 500.0 g water, what is the solution molality?
A) 3.56 m
B) 2.04 m
C) 1.78 m
D) 0.90 m
E) 0.250 m
A) 3.56 m
B) 2.04 m
C) 1.78 m
D) 0.90 m
E) 0.250 m

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
61
the van't Hoff i of HF differs from that of HCl?

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
62
The osmotic pressure of 1.00 g of a polymer dissolved in benzene to give 200 mL of solution is 1.50 kPa at 25 C .Estimate the average molar mass of the polymer.The gas law constant is 0.0821 L.atm.mol-1.K-1.
A) 41 300 g.mol-1
B) 8260 g.mol-1
C) 693 g.mol-1
D) 1650 g.mol-1
E) 62 000 g.mol-1
A) 41 300 g.mol-1
B) 8260 g.mol-1
C) 693 g.mol-1
D) 1650 g.mol-1
E) 62 000 g.mol-1

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
63
Solutions in which intermolecular forces are stronger in the solution than in the pure components have negative enthalpies of mixing.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the diagram below. 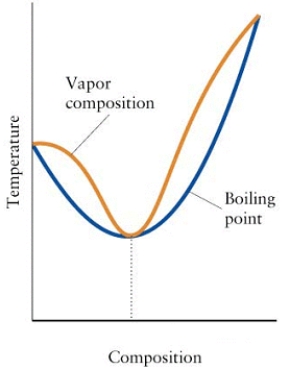
(a)What is the mixture in the diagram called?
(b)Can the components of this mixture be separated by fractional distillation? Explain.

(a)What is the mixture in the diagram called?
(b)Can the components of this mixture be separated by fractional distillation? Explain.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following pairs have a vant Hoff i factor of 3?
A) Sodium sulfate and potassium chloride
B) Calcium chloride and potassium sulfate
C) Magnesium sulfate and ethylene glycol
D) Glucose and sodium chloride
E) Perchloric acid and barium hydroxide
A) Sodium sulfate and potassium chloride
B) Calcium chloride and potassium sulfate
C) Magnesium sulfate and ethylene glycol
D) Glucose and sodium chloride
E) Perchloric acid and barium hydroxide

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
66
Blood, sweat,And tears are about 0.15 M in sodium chloride.Estimate the osmotic pressure of these solutions at 37 C.The gas constant is 0.0821 L.atm.mol-1.K-1.
A) 3.8 atm
B) 11 atm
C) 0.91 atm
D) 1.8 atm
E) 7.6 atm
A) 3.8 atm
B) 11 atm
C) 0.91 atm
D) 1.8 atm
E) 7.6 atm

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
67
Water and acetone,CH3COCH3,both freeze at a higher temperature under pressure.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
68
Of the following five materials, which has the lowest freezing point and the highest boiling point?
A) 1.5 m magnesium phosphate
B) 2.0 m potassium chloride
C) 1.0 m sodium chloride
D) 1.5 m aluminum nitrate
E) 1.5 m calcium chloride
A) 1.5 m magnesium phosphate
B) 2.0 m potassium chloride
C) 1.0 m sodium chloride
D) 1.5 m aluminum nitrate
E) 1.5 m calcium chloride

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
69
The critical temperature of N2 is -147oC.Nitrogen gas cannot be converted to liquid nitrogen at -73oC,even at extremely high pressures.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
70
The addition of 125 mg of caffeine to 100 g of cyclohexane lowered the freezing point by 0.13 K .Calculate the molar mass of caffeine.The kf for cyclohexane is 20.1 K.kg.mol-1.
A) 47.8 g.mol-1
B) 481 g.mol-1
C) 96.5 g.mol-1
D) 19.3 g.mol-1
E) 193 g.mol-1
A) 47.8 g.mol-1
B) 481 g.mol-1
C) 96.5 g.mol-1
D) 19.3 g.mol-1
E) 193 g.mol-1

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
71
The vapor pressures of pure carbon disulfide and carbon tetrachloride are 360 and 99.8 Torr, respectively,At 296 K.What is the vapor pressure of a solution containing 50.0 g of each compound?
A) 260 Torr
B) 274 Torr
C) 241 Torr
D) 33.0 Torr
E) 460 Torr
A) 260 Torr
B) 274 Torr
C) 241 Torr
D) 33.0 Torr
E) 460 Torr

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the phase diagram for CO2 in the text on page 315.A sample of carbon dioxide at 10 C and 10 atm is a liquid.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following pairs have a vant Hoff i factor of 2?
A) Sodium sulfate and potassium chloride
B) Sodium chloride and magnesium sulfate
C) Magnesium sulfate and ethylene glycol
D) Glucose and sodium chloride
E) Perchloric acid and barium hydroxide
A) Sodium sulfate and potassium chloride
B) Sodium chloride and magnesium sulfate
C) Magnesium sulfate and ethylene glycol
D) Glucose and sodium chloride
E) Perchloric acid and barium hydroxide

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
74
The freezing point of seawater is about -1.85 C .If seawater is an aqueous solution of sodium chloride,Calculate the molality of seawater.The kf for water is 1.86 K/m.
A) 1.99 m
B) 0.995 m
C) 3.44 m
D) 3.70 m
E) 0.497 m
A) 1.99 m
B) 0.995 m
C) 3.44 m
D) 3.70 m
E) 0.497 m

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
75
The vapor pressures of pure carbon disulfide and carbon tetrachloride are 360 and 99.8 Torr, respectively,At 296 K.What is the vapor pressure of a solution containing 50.0 g of each compound?
A) 241 Torr
B) 33.0 Torr
C) 260 Torr
D) 274 Torr
E) 460 Torr
A) 241 Torr
B) 33.0 Torr
C) 260 Torr
D) 274 Torr
E) 460 Torr

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following has the lowest freezing point?
A) 2.0 m sodium perchlorate
B) 1.0 m potassium phosphate
C) 1.5 m aluminum nitrate
D) 1.0 m aluminum sulfate
E) 1.5 m magnesium phosphate
A) 2.0 m sodium perchlorate
B) 1.0 m potassium phosphate
C) 1.5 m aluminum nitrate
D) 1.0 m aluminum sulfate
E) 1.5 m magnesium phosphate

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
77
Rank the following species in order of increasing vapor pressure at 25 C.
water,diethyl ether,ethanol,mercury
water,diethyl ether,ethanol,mercury

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
78
The normal boiling point of ethanol is 78 C.If the vapor pressure of ethanol is 13.3 kPa at 34.9 C,calculate the enthalpy of vaporization of ethanol.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
79
An animal cell assumes its normal volume when it is placed in a solution with a total solute molarity of 0.3 M .If the cell is placed in a solution with a total solute molarity of 0.1 M,
A) water enters the cell, causing expansion.
B) water leaves the cell, causing contraction.
C) the escaping tendency of water in the cell increases.
D) no movement of water takes place.
A) water enters the cell, causing expansion.
B) water leaves the cell, causing contraction.
C) the escaping tendency of water in the cell increases.
D) no movement of water takes place.

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following 1.0 M solutions contains the most particles?
A) Ethylene glycol
B) Potassium chloride
C) Magnesium sulfate
D) Glucose
E) Sodium sulfate
A) Ethylene glycol
B) Potassium chloride
C) Magnesium sulfate
D) Glucose
E) Sodium sulfate

Unlock Deck
Unlock for access to all 191 flashcards in this deck.
Unlock Deck
k this deck


