Deck 9: Molecular Geometry and Bonding Theories
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/183
Play
Full screen (f)
Deck 9: Molecular Geometry and Bonding Theories
1
Of the molecules below,only ________ is nonpolar.
A)BF3
B)NF3
C)IF3
D)PBr3
E)BrCl3
A)BF3
B)NF3
C)IF3
D)PBr3
E)BrCl3
BF3
2
The electron-domain geometry and the molecular geometry of a molecule of the general formula ABn will always be the same if ________.
A)there are no lone pairs on the central atom
B)there is more than one central atom
C)n is greater than four
D)n is less than four
E)the octet rule is obeyed
A)there are no lone pairs on the central atom
B)there is more than one central atom
C)n is greater than four
D)n is less than four
E)the octet rule is obeyed
there are no lone pairs on the central atom
3
An electron domain consists of ________.
a.a nonbonding pair of electrons
b.a single bond
c.a multiple bond
A)a only
B)b only
C)c only
D)a, b, and c
E)b and c
a.a nonbonding pair of electrons
b.a single bond
c.a multiple bond
A)a only
B)b only
C)c only
D)a, b, and c
E)b and c
a, b, and c
4
For molecules of the general formula ABn,n can be greater than four ________.
A)for any element A
B)only when A is an element from the third period or below the third period
C)only when A is boron or beryllium
D)only when A is carbon
E)only when A is Xe
A)for any element A
B)only when A is an element from the third period or below the third period
C)only when A is boron or beryllium
D)only when A is carbon
E)only when A is Xe

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
5
The molecular geometry of the BrO3- ion is ________.
A)trigonal pyramidal
B)trigonal planar
C)bent
D)tetrahedral
E)T-shaped
A)trigonal pyramidal
B)trigonal planar
C)bent
D)tetrahedral
E)T-shaped

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
6
For which of the molecules is the molecular geometry (shape)the same as the VSEPR electron domain arrangement (electron domain geometry)?
A)(i)and (ii)
B)(i)and (iii)
C)(ii)and (v)
D)(iv)and (v)
E)(v)only
A)(i)and (ii)
B)(i)and (iii)
C)(ii)and (v)
D)(iv)and (v)
E)(v)only

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
7
The bond angles marked a,b,and c in the molecule below are about ________,________,and ________,respectively. 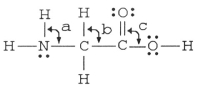
A)109.5°, 109.5°, 109.5°
B)120°, 109.5°, 120°
C)109.5°, 109.5°, 120°
D)90°, 180°, 90°
E)109.5°, 109.5°, 90°
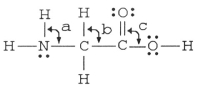
A)109.5°, 109.5°, 109.5°
B)120°, 109.5°, 120°
C)109.5°, 109.5°, 120°
D)90°, 180°, 90°
E)109.5°, 109.5°, 90°

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
8
The central Xe atom in the XeF4 molecule has ________ unbonded electron pair(s)and ________ bonded electron pair(s)in its valence shell.
A)1, 4
B)2, 4
C)4, 0
D)4, 1
E)4, 2
A)1, 4
B)2, 4
C)4, 0
D)4, 1
E)4, 2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
9
What is the molecular shape of H2O?
A)T-shaped
B)tetrahedral
C)linear
D)trigonal pyramidal
E)bent
A)T-shaped
B)tetrahedral
C)linear
D)trigonal pyramidal
E)bent

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
10
The basis of the VSEPR model of molecular bonding is ________.
A)regions of electron density on an atom will organize themselves so as to maximize s-character
B)regions of electron density in the valence shell of an atom will arrange themselves so as to maximize overlap
C)atomic orbitals of the bonding atoms must overlap for a bond to form
D)electron domains in the valence shell of an atom will arrange themselves so as to minimize repulsions
E)hybrid orbitals will form as necessary to, as closely as possible, achieve spherical symmetry
A)regions of electron density on an atom will organize themselves so as to maximize s-character
B)regions of electron density in the valence shell of an atom will arrange themselves so as to maximize overlap
C)atomic orbitals of the bonding atoms must overlap for a bond to form
D)electron domains in the valence shell of an atom will arrange themselves so as to minimize repulsions
E)hybrid orbitals will form as necessary to, as closely as possible, achieve spherical symmetry

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
11
Of the following species,________ will have bond angles of 120°.
A)PH3
B)ClF3
C)NCl3
D)BCl3
E)All of these will have bond angles of 120°.
A)PH3
B)ClF3
C)NCl3
D)BCl3
E)All of these will have bond angles of 120°.

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
12
The molecular geometry consists of ________.
a.a nonbonding pair of electrons
b.a single bond
c.a multiple bond
A)a only
B)b only
C)c only
D)a, b, and c
E)b and c
a.a nonbonding pair of electrons
b.a single bond
c.a multiple bond
A)a only
B)b only
C)c only
D)a, b, and c
E)b and c

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
13
The electron-domain geometry and the molecular geometry of a molecule of the general formula ABn are ________.
A)never the same
B)always the same
C)sometimes the same
D)not related
E)mirror images of one another
A)never the same
B)always the same
C)sometimes the same
D)not related
E)mirror images of one another

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
14
The molecular geometry of the right-most carbon in the molecule below is ________. 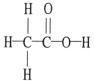
A)trigonal planar
B)trigonal bipyramidal
C)tetrahedral
D)octahedral
E)T-shaped

A)trigonal planar
B)trigonal bipyramidal
C)tetrahedral
D)octahedral
E)T-shaped

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
15
The electron-domain geometry of ________ is tetrahedral.
A)CBr4
B)PH3
C)CCl2Br2
D)XeF4
E)all of the above except XeF4
A)CBr4
B)PH3
C)CCl2Br2
D)XeF4
E)all of the above except XeF4

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
16
Of the molecules below,only ________ is polar.
A)CCl4
B)CH4
C)SeF4
D)SiCl4
A)CCl4
B)CH4
C)SeF4
D)SiCl4

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
17
The bond angles marked a,b,and c in the molecule below are about ________,________,and ________,respectively. 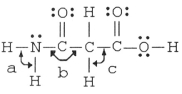
A)90°, 90°, 90°
B)120°, 120°, 90°
C)120°, 120°, 109.5°
D)109.5°, 120°, 109.5°
E)109.5°, 90°, 120°

A)90°, 90°, 90°
B)120°, 120°, 90°
C)120°, 120°, 109.5°
D)109.5°, 120°, 109.5°
E)109.5°, 90°, 120°

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
18
PCl5 has ________ electron domains and a ________ molecular arrangement.
A)6, trigonal bipyramidal
B)6, tetrahedral
C)5, square pyramidal
D)5, trigonal bipyramidal
E)6, seesaw
A)6, trigonal bipyramidal
B)6, tetrahedral
C)5, square pyramidal
D)5, trigonal bipyramidal
E)6, seesaw

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
19
In counting the electron domains around the central atom in VSEPR theory,a ________ is not included.
A)nonbonding pair of electrons
B)single covalent bond
C)core level electron pair
D)double covalent bond
E)triple covalent bond
A)nonbonding pair of electrons
B)single covalent bond
C)core level electron pair
D)double covalent bond
E)triple covalent bond

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
20
The molecular geometry of the left-most carbon atom in the molecule below is ________. 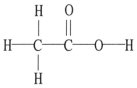
A)trigonal planar
B)trigonal bipyramidal
C)tetrahedral
D)octahedral
E)T-shaped

A)trigonal planar
B)trigonal bipyramidal
C)tetrahedral
D)octahedral
E)T-shaped

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
21
The hybrid orbitals used for bonding by the sulfur atom in the SF4 molecule are ________ orbitals.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
22
For molecules with only one central atom,how many lone pairs on the central atom guarantees molecular polarity?
A)1
B)2
C)1 or 2
D)3
E)1 or 3
A)1
B)2
C)1 or 2
D)3
E)1 or 3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
23
The molecular geometry of the CHF3 molecule is ________,and the molecule is ________.
A)trigonal pyramidal, polar
B)tetrahedral, nonpolar
C)seesaw, nonpolar
D)tetrahedral, polar
E)seesaw, polar
A)trigonal pyramidal, polar
B)tetrahedral, nonpolar
C)seesaw, nonpolar
D)tetrahedral, polar
E)seesaw, polar

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
24
The sp3d2 atomic hybrid orbital set accommodates ________ electron domains.
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
25
What are the hybrid orbitals used for bonding by Xe in a XeCl4 molecule?
A)sp2
B)sp3d2
C)sp3
D)sp3d
E)sp
A)sp2
B)sp3d2
C)sp3
D)sp3d
E)sp

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
26
What is the hybridization of the carbon atom attached to the two oxygen atoms in the structure below? 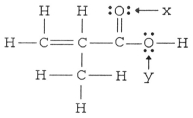
A)sp3
B)sp
C)sp3d2
D)sp2
E)sp3d

A)sp3
B)sp
C)sp3d2
D)sp2
E)sp3d

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the molecules has a square planar shape?
A)(i)and (ii)
B)(i)and (v)
C)(iii)only
D)(ii)and (iv)
E)(iv)only
A)(i)and (ii)
B)(i)and (v)
C)(iii)only
D)(ii)and (iv)
E)(iv)only

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
28
Of the following molecules,only ________ is polar.
A)CCl4
B)BCl3
C)NCl3
D)BeCl2
E)Cl2
A)CCl4
B)BCl3
C)NCl3
D)BeCl2
E)Cl2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
29
The molecular geometry of the PF3 molecule is ________,and this molecule is ________.
A)trigonal planar, polar
B)trigonal planar, nonpolar
C)trigonal pyramidal, polar
D)trigonal pyramidal, nonpolar
E)tetrahedral, unipolar
A)trigonal planar, polar
B)trigonal planar, nonpolar
C)trigonal pyramidal, polar
D)trigonal pyramidal, nonpolar
E)tetrahedral, unipolar

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
30
The hybridizations of nitrogen in NF3 and NH3 are ________ and ________,respectively.
A)sp2, sp2
B)sp, sp3
C)sp3, sp
D)sp3, sp3
E)sp2, sp3
A)sp2, sp2
B)sp, sp3
C)sp3, sp
D)sp3, sp3
E)sp2, sp3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
31
The sp2 atomic hybrid orbital set accommodates ________ electron domains.
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
32
Of the following molecules,only ________ is polar.
A)BeCl2
B)BF3
C)C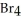
D)SiH2Cl2
E)Cl2
A)BeCl2
B)BF3
C)C
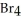
D)SiH2Cl2
E)Cl2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the molecules has a see-saw shape?
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)
A)(i)
B)(ii)
C)(iii)
D)(iv)
E)(v)

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
34
Three monosulfur fluorides are observed: SF2,SF4,and SF6.Of these,________ is/are polar.
A)SF2 only
B)SF2 and SF4 only
C)SF4 only
D)SF6 only
E)SF2, SF4, and SF6
A)SF2 only
B)SF2 and SF4 only
C)SF4 only
D)SF6 only
E)SF2, SF4, and SF6

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
35
The hybridizations of iodine in IF3 and IF5 are ________ and ________,respectively.
A)sp3, sp3d
B)sp3d, sp3d2
C)sp3d, sp3
D)sp3d2, sp3d
E)sp3d2, sp3d2
A)sp3, sp3d
B)sp3d, sp3d2
C)sp3d, sp3
D)sp3d2, sp3d
E)sp3d2, sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
36
Of the following,the central atom is sp3d2 hybridized only in ________.
A)PCl5
B)XeF4
C)PH3
D)Br3-
E)BeF2
A)PCl5
B)XeF4
C)PH3
D)Br3-
E)BeF2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
37
The molecular geometry of the BCl3 molecule is ________,and this molecule is ________.
A)trigonal pyramidal, polar
B)trigonal pyramidal, nonpolar
C)trigonal planar, polar
D)trigonal planar, nonpolar
E)trigonal bipyramidal, polar
A)trigonal pyramidal, polar
B)trigonal pyramidal, nonpolar
C)trigonal planar, polar
D)trigonal planar, nonpolar
E)trigonal bipyramidal, polar

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
38
The hybridization scheme for BeF2 is ________.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
39
The hybridizations of bromine in BrF5 and of arsenic in AsF5 are ________ and ________,respectively.
A)sp3, sp3d
B)sp3d, sp3d2
C)sp3d, sp3
D)sp3d2, sp3d
E)sp3d2, sp3d2
A)sp3, sp3d
B)sp3d, sp3d2
C)sp3d, sp3
D)sp3d2, sp3d
E)sp3d2, sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
40
The combination of two atomic orbitals results in the formation of ________ molecular orbitals.
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
41
The hybridization of nitrogen in the H-C  N: molecule is ________.
N: molecule is ________.
A)sp
B)s2p
C)s3p
D)sp2
E)sp3
 N: molecule is ________.
N: molecule is ________.A)sp
B)s2p
C)s3p
D)sp2
E)sp3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
42
________ hybrid orbitals are used for bonding by Xe in the XeF4 molecule.
A)sp2
B)sp3
C)sp3d
D)sp3d2
E)sp
A)sp2
B)sp3
C)sp3d
D)sp3d2
E)sp

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
43
A molecule must have at least two resonance structures in order to display ________.
A)delocalized σ bonding
B)trigonal planar electron group geometry
C)localized π bonding
D)delocalized π bonding
E)localized σ bonding
A)delocalized σ bonding
B)trigonal planar electron group geometry
C)localized π bonding
D)delocalized π bonding
E)localized σ bonding

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
44
There are ________ σ bonds and ________ π bonds in H3C-CH2-CH  CH-CH2-C
CH-CH2-C  CH.
CH.
A)14, 2
B)10, 3
C)12, 2
D)13, 2
E)16, 3
 CH-CH2-C
CH-CH2-C  CH.
CH.A)14, 2
B)10, 3
C)12, 2
D)13, 2
E)16, 3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
45
What is the hybridization of the I atom in a IF5 molecule?
A)sp2d2
B)sp3d
C)sp3
D)sp3d2
E)sp2
A)sp2d2
B)sp3d
C)sp3
D)sp3d2
E)sp2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
46
A typical triple bond ________.
A)consists of one σ bond and two π bonds
B)consists of three shared electrons
C)consists of two σ bonds and one π bond
D)consists of six shared electron pairs
E)is longer than a single bond
A)consists of one σ bond and two π bonds
B)consists of three shared electrons
C)consists of two σ bonds and one π bond
D)consists of six shared electron pairs
E)is longer than a single bond

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
47
The π bond in ethylene,H2C  CH2,results from the overlap of ________.
CH2,results from the overlap of ________.
A)sp3 hybrid orbitals
B)s atomic orbitals
C)sp hybrid orbitals
D)sp2 hybrid orbitals
E)p atomic orbitals
 CH2,results from the overlap of ________.
CH2,results from the overlap of ________.A)sp3 hybrid orbitals
B)s atomic orbitals
C)sp hybrid orbitals
D)sp2 hybrid orbitals
E)p atomic orbitals

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
48
A triatomic molecule cannot be linear if the hybridization of the central atoms is ________.
A)sp
B)sp2
C)sp3
D)sp2 or sp3
E)sp2d or sp3d2
A)sp
B)sp2
C)sp3
D)sp2 or sp3
E)sp2d or sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
49
The N-H bond in ammonia consists of ________.
A)one σ bond and one π bond
B)one σ bond and no π bonds
C)one σ bond and two π bonds
D)two σ bonds and one π bond
E)two σ bonds and two π bonds
A)one σ bond and one π bond
B)one σ bond and no π bonds
C)one σ bond and two π bonds
D)two σ bonds and one π bond
E)two σ bonds and two π bonds

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
50
The carbon-hydrogen σ bond in ethylene,H2C  CH2,results from the overlap of ________.
CH2,results from the overlap of ________.
A)sp2-hybrid and s-atomic orbitals
B)sp hybrid orbitals
C)sp3 hybrid orbitals
D)s-hybrid and sp2-atomic orbitals
E)s atomic orbitals
 CH2,results from the overlap of ________.
CH2,results from the overlap of ________.A)sp2-hybrid and s-atomic orbitals
B)sp hybrid orbitals
C)sp3 hybrid orbitals
D)s-hybrid and sp2-atomic orbitals
E)s atomic orbitals

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
51
Valence bond theory addresses all of the following except ________.
A)molecular shape
B)covalent bonding
C)excited states of molecules
D)hybridization
E)multiple bonds
A)molecular shape
B)covalent bonding
C)excited states of molecules
D)hybridization
E)multiple bonds

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
52
In a C=C bond,the σ bond results from overlap of ________ orbitals and the π bond(s)result from overlap of ________ orbitals.
A)sp2-hybrid, p-atomic
B)sp2-atomic, p-hybrid
C)sp3-hybrid, p-atomic
D)sp-hybrid, p-atomic
E)σ-atomic, π-hybrid
A)sp2-hybrid, p-atomic
B)sp2-atomic, p-hybrid
C)sp3-hybrid, p-atomic
D)sp-hybrid, p-atomic
E)σ-atomic, π-hybrid

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
53
In which of the molecules is the central atom sp2 hybridized?
A)(i)only
B)(i)and (ii)
C)(iii)and (iv)
D)(iii)only
E)(iv)and (v)
A)(i)only
B)(i)and (ii)
C)(iii)and (iv)
D)(iii)only
E)(iv)and (v)

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following molecules or ions have various resonance structures?
CO2 O3 CO32-
A)CO2, O3, and CO32-
B)CO2 and O3
C)O3 and CO32-
D)CO32- only
E)None of the above will exhibit delocalized bonding.
CO2 O3 CO32-
A)CO2, O3, and CO32-
B)CO2 and O3
C)O3 and CO32-
D)CO32- only
E)None of the above will exhibit delocalized bonding.

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following molecules or ions will exhibit delocalized bonding?
NO2- NH4+ N3-
A)NH4+ and N3-
B)NO2- only
C)NO2-, NH4+, and N3-
D)N3- only
E)NO2- and N3-
NO2- NH4+ N3-
A)NH4+ and N3-
B)NO2- only
C)NO2-, NH4+, and N3-
D)N3- only
E)NO2- and N3-

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
56
In a SO42- ion,"localized" bonding electrons are associated with ________ particular atoms.
A)zero
B)one
C)two
D)three
E)four
A)zero
B)one
C)two
D)three
E)four

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
57
The hybridization of the terminal carbons in the H2C  C
C  CH2 molecule is ________.
CH2 molecule is ________.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
 C
C  CH2 molecule is ________.
CH2 molecule is ________.A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
58
A typical double bond consists of ________.
A)three sigma bonds
B)three pi bonds
C)one sigma and two pi bonds
D)one sigma and one pi bond
E)three ionic bonds
A)three sigma bonds
B)three pi bonds
C)one sigma and two pi bonds
D)one sigma and one pi bond
E)three ionic bonds

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
59
When four atomic orbitals are mixed to form hybrid orbitals,how many hybrid orbitals are formed?
A)one
B)six
C)three
D)four
E)five
A)one
B)six
C)three
D)four
E)five

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
60
A typical double bond ________.
A)is stronger and shorter than a single bond
B)consists of one σ bond and one π bond
C)imparts rigidity to a molecule
D)consists of two shared electron pairs
E)All of the above answers are correct.
A)is stronger and shorter than a single bond
B)consists of one σ bond and one π bond
C)imparts rigidity to a molecule
D)consists of two shared electron pairs
E)All of the above answers are correct.

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
61
Molecular Orbital theory correctly predicts paramagnetism of oxygen gas,O2.This is because ________.
A)the bond order in O2 can be shown to be equal to 2
B)there are more electrons in the bonding orbitals than in the antibonding orbitals
C)the energy of the π2p MOs is higher than that of the σ2p MO
D)there are two unpaired electrons in the MO electron configuration of O2
E)the O-O bond distance is relatively short
A)the bond order in O2 can be shown to be equal to 2
B)there are more electrons in the bonding orbitals than in the antibonding orbitals
C)the energy of the π2p MOs is higher than that of the σ2p MO
D)there are two unpaired electrons in the MO electron configuration of O2
E)the O-O bond distance is relatively short

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
62
The hybridization of carbon in the H-C  C-H molecule is ________.
C-H molecule is ________.
A)sp2
B)sp
C)s2p
D)s3p
E)sp3
 C-H molecule is ________.
C-H molecule is ________.A)sp2
B)sp
C)s2p
D)s3p
E)sp3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
63
Based on molecular orbital theory,the bond orders of the H-H bonds in H2,H2+,and H2- are ________,respectively
A)1, 0, and 0
B)1, 1/2, and 0
C)1, 0, and 1/2
D)1, 1/2, and 1/2
E)1, 2, and 0
A)1, 0, and 0
B)1, 1/2, and 0
C)1, 0, and 1/2
D)1, 1/2, and 1/2
E)1, 2, and 0

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
64
An antibonding π orbital contains a maximum of ________ electrons.
A)1
B)2
C)4
D)6
E)8
A)1
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
65
Electrons in ________ bonds remain localized between two atoms.Electrons in ________ bonds can become delocalized between more than two atoms.
A)pi, sigma
B)sigma, pi
C)pi, pi
D)sigma, sigma
E)ionic, sigma
A)pi, sigma
B)sigma, pi
C)pi, pi
D)sigma, sigma
E)ionic, sigma

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
66
The hybridization of the oxygen atom labeled x in the structure below is ________. 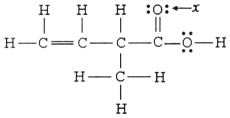
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
67
Based on molecular orbital theory,the bond order of the H-H bond in the H2+ ion is ________.
A)0
B)1/2
C)1
D)3/2
E)2
A)0
B)1/2
C)1
D)3/2
E)2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
68
The bond order of any molecule containing equal numbers of bonding and antibonding electrons is ________.
A)0
B)1
C)2
D)3
E)1/2
A)0
B)1
C)2
D)3
E)1/2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
69
The hybridization of the carbon atom labeled x in the molecule below is ________. 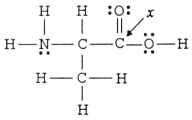
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
70
In comparing the same two atoms bonded together,the ________ the bond order,the ________ the bond length,and the ________ the bond energy.
A)greater, shorter, greater
B)greater, greater, greater
C)greater, longer, greater
D)greater, greater, smaller
E)smaller, greater, greater
A)greater, shorter, greater
B)greater, greater, greater
C)greater, longer, greater
D)greater, greater, smaller
E)smaller, greater, greater

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
71
Based on molecular orbital theory,the only molecule in the list below that has unpaired electrons is ________.
A)C2
B)N2
C)F2
D)O2
E)Li2
A)C2
B)N2
C)F2
D)O2
E)Li2

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
72
According to molecular orbital theory,how many unpaired electrons are in a peroxide ion,O22-?
A)0
B)1/2
C)1
D)2
E)3
A)0
B)1/2
C)1
D)2
E)3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
73
Structural changes around a ________ bond in the retinal portion of the rhodopsin molecule trigger the chemical reactions that result in vision.
A)C=C
B)C-C
C)C C
C
D)C-H
E)C=N
A)C=C
B)C-C
C)C
 C
CD)C-H
E)C=N

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
74
According to MO theory,overlap of two s atomic orbitals produces ________.
A)one bonding molecular orbital and one hybrid orbital
B)two bonding molecular orbitals
C)two bonding molecular orbitals and two antibonding molecular orbitals
D)two bonding molecular orbitals and one antibonding molecular orbital
E)one bonding molecular orbital and one antibonding molecular orbital
A)one bonding molecular orbital and one hybrid orbital
B)two bonding molecular orbitals
C)two bonding molecular orbitals and two antibonding molecular orbitals
D)two bonding molecular orbitals and one antibonding molecular orbital
E)one bonding molecular orbital and one antibonding molecular orbital

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
75
Molecular Orbital theory correctly predicts diamagnetism of fluorine gas,F2.This is because ________.
A)the bond order in F2 can be shown to be equal to 1
B)there are more electrons in the bonding orbitals than in the antibonding orbitals
C)all electrons in the MO electron configuration of F2 are paired
D)the energy of the π2pMOs is higher than that of the σ2p MO
E)the F-F bond enthalpy is very low
A)the bond order in F2 can be shown to be equal to 1
B)there are more electrons in the bonding orbitals than in the antibonding orbitals
C)all electrons in the MO electron configuration of F2 are paired
D)the energy of the π2pMOs is higher than that of the σ2p MO
E)the F-F bond enthalpy is very low

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
76
According to molecular orbital theory,the bond order of N-N in nitrogen gas is ________.
A)0
B)1/2
C)1
D)2
E)3
A)0
B)1/2
C)1
D)2
E)3

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
77
A molecular orbital can accommodate a maximum of ________ electron(s).
A)1
B)2
C)4
D)6
E)12
A)1
B)2
C)4
D)6
E)12

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
78
The hybridization and molecular shape of the carbon atom in carbon dioxide is ________.
A)sp and linear
B)sp3 and bent
C)sp2 and linear
D)sp2d and bent
E)sp2d2 and linear
A)sp and linear
B)sp3 and bent
C)sp2 and linear
D)sp2d and bent
E)sp2d2 and linear

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
79
In comparing the same two atoms bonded together,the ________ the bond order,the ________ the bond length,and the ________ the bond energy.
A)greater, shorter, greater
B)greater, greater, greater
C)greater, longer, greater
D)smaller, longer, smaller
E)smaller, greater, greater
A)greater, shorter, greater
B)greater, greater, greater
C)greater, longer, greater
D)smaller, longer, smaller
E)smaller, greater, greater

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck
80
According to molecular orbital theory,the bond order of He-He in the He2 molecule is ________.
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 183 flashcards in this deck.
Unlock Deck
k this deck


