Deck 11: Liquids and Intermolecular Forces
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/124
Play
Full screen (f)
Deck 11: Liquids and Intermolecular Forces
1
Hydrogen bonding is a special case of ________.
A)London-dispersion forces
B)ion-dipole attraction
C)dipole-dipole attractions
D)ion-ion interactions
E)none of the above
A)London-dispersion forces
B)ion-dipole attraction
C)dipole-dipole attractions
D)ion-ion interactions
E)none of the above
dipole-dipole attractions
2
Which species has London dispersion forces as the only intermolecular force?
A)KBr
B)HI
C)CH3OH
D)CH3CH3
E)CH3F
A)KBr
B)HI
C)CH3OH
D)CH3CH3
E)CH3F
CH3CH3
3
________ are particularly polarizable.
A)Small nonpolar molecules
B)Small polar molecules
C)Large nonpolar molecules
D)Large polar molecules
E)Large molecules, regardless of their polarity,
A)Small nonpolar molecules
B)Small polar molecules
C)Large nonpolar molecules
D)Large polar molecules
E)Large molecules, regardless of their polarity,
Large molecules, regardless of their polarity,
4
The strongest interparticle attractions exist between particles of a ________,and the weakest interparticle attractions exist between particles of a ________.
A)solid, liquid
B)solid, gas
C)liquid, gas
D)liquid, solid
E)gas, solid
A)solid, liquid
B)solid, gas
C)liquid, gas
D)liquid, solid
E)gas, solid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
5
Crystalline solids ________.
A)have their particles arranged randomly
B)have ordered structures
C)are usually very soft
D)exist only at high temperatures
E)exist only at very low temperatures
A)have their particles arranged randomly
B)have ordered structures
C)are usually very soft
D)exist only at high temperatures
E)exist only at very low temperatures

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
6
In liquids,the attractive intermolecular forces are ________.
A)very weak compared with kinetic energies of the molecules
B)strong enough to hold molecules relatively close together
C)strong enough to keep the molecules confined to vibrating about their fixed lattice points
D)not strong enough to keep molecules from moving past each other
E)strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other
A)very weak compared with kinetic energies of the molecules
B)strong enough to hold molecules relatively close together
C)strong enough to keep the molecules confined to vibrating about their fixed lattice points
D)not strong enough to keep molecules from moving past each other
E)strong enough to hold molecules relatively close together but not strong enough to keep molecules from moving past each other

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
7
The intermolecular force(s)responsible for the fact that CH4 has the lowest boiling point in the set CH4,CH3CH3,CH3CH2CH3,CH3CH2CH2CH3 is/are ________.
A)hydrogen bonding
B)London dispersion forces
C)mainly hydrogen bonding but also dipole-dipole interactions
D)dipole-dipole interactions
E)mainly London-dispersion forces but also dipole-dipole interactions
A)hydrogen bonding
B)London dispersion forces
C)mainly hydrogen bonding but also dipole-dipole interactions
D)dipole-dipole interactions
E)mainly London-dispersion forces but also dipole-dipole interactions

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
8
Which species has London dispersion forces as the only intermolecular force?
A)CH3CH2OH
B)Ar
C)NH3
D)HBr
E)H2O
A)CH3CH2OH
B)Ar
C)NH3
D)HBr
E)H2O

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
9
Which statement is true about liquids but not true about solids?
A)They flow and are highly ordered.
B)They are highly ordered and not compressible.
C)They flow and are compressible.
D)They assume both the volume and the shape of their containers.
E)They flow and are not compressible.
A)They flow and are highly ordered.
B)They are highly ordered and not compressible.
C)They flow and are compressible.
D)They assume both the volume and the shape of their containers.
E)They flow and are not compressible.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
10
The ease with which the charge distribution in a molecule can be distorted by an external electrical field is called the ________.
A)electronegativity
B)hydrogen bonding
C)polarizability
D)volatility
E)viscosity
A)electronegativity
B)hydrogen bonding
C)polarizability
D)volatility
E)viscosity

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
11
As a gaseous element condenses,the atoms become ________ and they have ________ attraction for one another.
A)more separated, more
B)more separated, less
C)closer together, more
D)closer together, less
E)larger, greater
A)more separated, more
B)more separated, less
C)closer together, more
D)closer together, less
E)larger, greater

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
12
Which molecule has hydrogen bonding as the predominant intermolecular force?
A)CH4
B)C6H6
C)CH3OH
D)CO2
E)C4H10
A)CH4
B)C6H6
C)CH3OH
D)CO2
E)C4H10

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
13
Together,liquids and solids constitute ________ phases of matter.
A)the compressible
B)the fluid
C)the condensed
D)all of the
E)the disordered
A)the compressible
B)the fluid
C)the condensed
D)all of the
E)the disordered

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
14
The predominant intermolecular force in water is ________.
A)London dispersion forces
B)hydrogen bonding
C)ion-dipole forces
D)dipole-dipole forces
E)ionic bonding
A)London dispersion forces
B)hydrogen bonding
C)ion-dipole forces
D)dipole-dipole forces
E)ionic bonding

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following substances will have hydrogen bonding as one of its intermolecular forces?
A)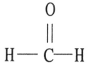
B)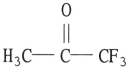
C)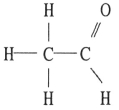
D)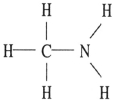
E)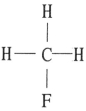
A)
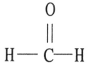
B)
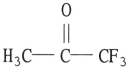
C)

D)

E)
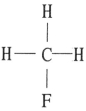

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
16
Elemental iodine (I2)is a solid at room temperature.What is the major attractive force that exists among different I2 molecules in the solid?
A)London dispersion forces
B)dipole-dipole interactions
C)ionic-dipole interactions
D)covalent-ionic interactions
E)dipole-dipole attractions
A)London dispersion forces
B)dipole-dipole interactions
C)ionic-dipole interactions
D)covalent-ionic interactions
E)dipole-dipole attractions

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following substances will not have hydrogen bonding as one of its intermolecular forces?
A)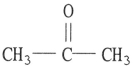
B)
C)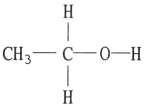
D)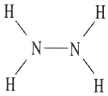
E)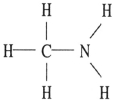
A)
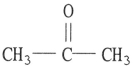
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
18
What intermolecular force is responsible for the fact that ice is less dense than liquid water?
A)London dispersion forces
B)dipole-dipole forces
C)ion-dipole forces
D)hydrogen bonding
E)ionic bonding
A)London dispersion forces
B)dipole-dipole forces
C)ion-dipole forces
D)hydrogen bonding
E)ionic bonding

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
19
A gas is ________ and assumes ________ of its container,whereas a liquid is ________ and assumes ________ of its container.
A)compressible, the volume and shape, not compressible, the shape of a portion
B)compressible, the shape, not compressible, the volume and shape
C)compressible, the volume and shape, compressible, the volume
D)condensed, the volume and shape, condensed, the volume and shape
E)condensed, the shape, compressible, the volume and shape
A)compressible, the volume and shape, not compressible, the shape of a portion
B)compressible, the shape, not compressible, the volume and shape
C)compressible, the volume and shape, compressible, the volume
D)condensed, the volume and shape, condensed, the volume and shape
E)condensed, the shape, compressible, the volume and shape

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
20
When KBr dissolves in water,aqueous K+ and Br- ions result.The force of attraction that exists between K+ and H2O is called a(n)________ interaction.
A)dipole-dipole
B)ion-dipole
C)ion-ion
D)London dispersion force
E)hydrogen bonding
A)dipole-dipole
B)ion-dipole
C)ion-ion
D)London dispersion force
E)hydrogen bonding

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements is false?
A)The absolute value of the heat of sublimation is equal to the absolute value of the heat of deposition.
B)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of deposition.
C)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of freezing.
D)The absolute value of the heat of sublimation is equal to the absolute value of the sum of the heat of condensation and the heat of freezing.
E)The absolute value of the heat of deposition is equal to sum of the absolute value of the heat of vaporization and the absolute value of the heat of freezing.
A)The absolute value of the heat of sublimation is equal to the absolute value of the heat of deposition.
B)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of deposition.
C)The heat of sublimation is equal to the sum of the heat of vaporization and the heat of freezing.
D)The absolute value of the heat of sublimation is equal to the absolute value of the sum of the heat of condensation and the heat of freezing.
E)The absolute value of the heat of deposition is equal to sum of the absolute value of the heat of vaporization and the absolute value of the heat of freezing.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
22
Viscosity is ________.
A)the "skin" on a liquid surface caused by intermolecular attraction
B)the resistance to flow
C)the same as density
D)inversely proportional to molar mass
E)unaffected by temperature
A)the "skin" on a liquid surface caused by intermolecular attraction
B)the resistance to flow
C)the same as density
D)inversely proportional to molar mass
E)unaffected by temperature

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
23
What types of intermolecular forces exist between water and HF?
A)dispersion forces and dipole-dipole forces
B)dispersion forces, dipole-dipole forces, and hydrogen bonds
C)dispersion forces and hydrogen bonds
D)dispersion forces
E)dispersion forces, hydrogen bonds, and ion-dipole forces
A)dispersion forces and dipole-dipole forces
B)dispersion forces, dipole-dipole forces, and hydrogen bonds
C)dispersion forces and hydrogen bonds
D)dispersion forces
E)dispersion forces, hydrogen bonds, and ion-dipole forces

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
24
The ________ (is)are associated with the heat energy being used up to increase distances between molecules.
A)phase change B → C
B)phase changes B → C and D → E
C)phase change D → E
D)phase change B → E
E)phase change C → E
A)phase change B → C
B)phase changes B → C and D → E
C)phase change D → E
D)phase change B → E
E)phase change C → E

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
25
Large intermolecular forces in a substance are manifested by ________.
A)low vapor pressure
B)high boiling point
C)high heats of fusion and vaporization
D)high critical temperatures and pressures
E)all of the above
A)low vapor pressure
B)high boiling point
C)high heats of fusion and vaporization
D)high critical temperatures and pressures
E)all of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
26
The shape of a liquid's meniscus is determined by ________.
A)the viscosity of the liquid
B)the type of material the container is made of
C)the relative magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and its container
D)the amount of hydrogen bonding in the liquid
E)the volume of the liquid
A)the viscosity of the liquid
B)the type of material the container is made of
C)the relative magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and its container
D)the amount of hydrogen bonding in the liquid
E)the volume of the liquid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
27
How high a liquid will rise up a narrow tube as a result of capillary action depends on ________.
A)the magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and the tube, and gravity
B)gravity alone
C)only the magnitude of adhesive forces between the liquid and the tube
D)the viscosity of the liquid
E)only the magnitude of cohesive forces in the liquid
A)the magnitudes of cohesive forces in the liquid and adhesive forces between the liquid and the tube, and gravity
B)gravity alone
C)only the magnitude of adhesive forces between the liquid and the tube
D)the viscosity of the liquid
E)only the magnitude of cohesive forces in the liquid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
28
The property responsible for the "beading up" of water is ________.
A)density
B)viscosity
C)vapor pressure
D)surface tension
E)hydrogen bonding
A)density
B)viscosity
C)vapor pressure
D)surface tension
E)hydrogen bonding

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
29
________ is the energy required to expand the surface area of a liquid by a unit amount of area.
A)Viscosity
B)Surface tension
C)Volatility
D)Meniscus
E)Capillary action
A)Viscosity
B)Surface tension
C)Volatility
D)Meniscus
E)Capillary action

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
30
Which compound has the strongest intermolecular forces?
A)CCl4
B)CI4
C)CH4
D)H2
E)O2
A)CCl4
B)CI4
C)CH4
D)H2
E)O2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
31
Based on the following information,which compound has the strongest intermolecular forces?
Substance ΔHvap (kJ/mol)
Argon (Ar)6.3
Benzene (C6H6)31.0
Ethanol (C2H5OH)39.3
Water (H2O)40.8
Methane (CH4)9.2
A)Argon
B)Benzene
C)Ethanol
D)Water
E)Methane
Substance ΔHvap (kJ/mol)
Argon (Ar)6.3
Benzene (C6H6)31.0
Ethanol (C2H5OH)39.3
Water (H2O)40.8
Methane (CH4)9.2
A)Argon
B)Benzene
C)Ethanol
D)Water
E)Methane

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following molecules has hydrogen bonding as its only intermolecular force?
A)HCl
B)NH3
C)H2O
D)CH3OH
E)None, all of the above exhibit dispersion forces.
A)HCl
B)NH3
C)H2O
D)CH3OH
E)None, all of the above exhibit dispersion forces.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
33
What types of intermolecular forces exist between CH3OH and H2O?
A)dipole-dipole and ion-dipole
B)dispersion forces, dipole-dipole, and hydrogen bonding
C)dispersion forces, dipole-dipole, and ion-dipole
D)dispersion forces, hydrogen bonding, dipole-dipole, and ion-dipole
E)dispersion forces and ion-dipole
A)dipole-dipole and ion-dipole
B)dispersion forces, dipole-dipole, and hydrogen bonding
C)dispersion forces, dipole-dipole, and ion-dipole
D)dispersion forces, hydrogen bonding, dipole-dipole, and ion-dipole
E)dispersion forces and ion-dipole

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
34
What type(s)of intermolecular forces exist between Cl2 and CCl4?
A)dispersion forces and ion-dipole
B)dispersion forces and dipole-dipole
C)dispersion forces
D)dispersion forces, ion-dipole, and dipole-dipole
E)None. Since both are gases at room temperature, they do not interact with each other.
A)dispersion forces and ion-dipole
B)dispersion forces and dipole-dipole
C)dispersion forces
D)dispersion forces, ion-dipole, and dipole-dipole
E)None. Since both are gases at room temperature, they do not interact with each other.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
35
Heat of sublimation can be approximated by adding together ________ and ________.
A)heat of fusion, heat of condensation
B)heat of fusion, heat of vaporization
C)heat of freezing (solidification), heat of condensation
D)heat of freezing (solidification), heat of vaporization
E)heat of deposition, heat of vaporization
A)heat of fusion, heat of condensation
B)heat of fusion, heat of vaporization
C)heat of freezing (solidification), heat of condensation
D)heat of freezing (solidification), heat of vaporization
E)heat of deposition, heat of vaporization

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules has London Forces as its only intermolecular force?
A)H2O
B)CH3CH2NH2
C)HOCH2CH2OH
D)CH3CH3
E)None, all of the above exhibit dispersion forces.
A)H2O
B)CH3CH2NH2
C)HOCH2CH2OH
D)CH3CH3
E)None, all of the above exhibit dispersion forces.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
37
What types of intermolecular forces exist between NH3 and H2O?
A)dispersion forces
B)dispersion forces and hydrogen bonds
C)dispersion forces and ion-dipole forces
D)dispersion forces, dipole-dipole forces, and hydrogen bonds
E)dispersion forces, hydrogen bonds, and ion-dipole forces
A)dispersion forces
B)dispersion forces and hydrogen bonds
C)dispersion forces and ion-dipole forces
D)dispersion forces, dipole-dipole forces, and hydrogen bonds
E)dispersion forces, hydrogen bonds, and ion-dipole forces

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
38
Which statements about viscosity are true?
(i)Viscosity increases as temperature decreases.
(ii)Viscosity increases as molecular weight increases.
(iii)Viscosity increases as intermolecular forces increase.
A)(i)only
B)(ii)and (iii)
C)(i)and (iii)
D)none
E)all
(i)Viscosity increases as temperature decreases.
(ii)Viscosity increases as molecular weight increases.
(iii)Viscosity increases as intermolecular forces increase.
A)(i)only
B)(ii)and (iii)
C)(i)and (iii)
D)none
E)all

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
39
Octane C8H18 molecules are held together by ________.
A)ion-ion interactions
B)hydrogen bonding
C)ion-dipole interactions
D)London dispersion forces
E)dipole-dipole interactions
A)ion-ion interactions
B)hydrogen bonding
C)ion-dipole interactions
D)London dispersion forces
E)dipole-dipole interactions

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
40
What type(s)of intermolecular forces exist between NH3 and PO43-?
A)dispersion forces
B)dispersion forces, ion-dipole, and dipole-dipole
C)dispersion forces and dipole-dipole
D)dispersion forces and ion-dipole
E)dispersion forces, ion-dipole, dipole-dipole, and hydrogen bonds
A)dispersion forces
B)dispersion forces, ion-dipole, and dipole-dipole
C)dispersion forces and dipole-dipole
D)dispersion forces and ion-dipole
E)dispersion forces, ion-dipole, dipole-dipole, and hydrogen bonds

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
41
In the ________ liquid-crystalline phase,the molecules are arranged in sheets,with their long axes parallel and their ends aligned as well.
A)smectic B
B)smectic C
C)smectic A
D)smectic E
E)smectic D
A)smectic B
B)smectic C
C)smectic A
D)smectic E
E)smectic D

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
42
A supercritical fluid can expand like a ________ to fill a container and has a density similar to that of a ________ so can behave as a solvent.
A)gas, plasma
B)gas, solid
C)solid, gas
D)liquid, gas
E)gas, liquid
A)gas, plasma
B)gas, solid
C)solid, gas
D)liquid, gas
E)gas, liquid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
43
Some things take longer to cook at high altitudes than at low altitudes because ________.
A)water boils at a lower temperature at high altitude than at low altitude
B)water boils at a higher temperature at high altitude than at low altitude
C)heat isn't conducted as well in low density air
D)natural gas flames don't burn as hot at high altitudes
E)there is a higher moisture content in the air at high altitude
A)water boils at a lower temperature at high altitude than at low altitude
B)water boils at a higher temperature at high altitude than at low altitude
C)heat isn't conducted as well in low density air
D)natural gas flames don't burn as hot at high altitudes
E)there is a higher moisture content in the air at high altitude

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
44
On a phase diagram,the melting point is the same as ________.
A)the triple point
B)the critical point
C)the freezing point
D)the boiling point
E)the vapor-pressure curve
A)the triple point
B)the critical point
C)the freezing point
D)the boiling point
E)the vapor-pressure curve

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
45
When the phase diagram for a substance has a solid-liquid phase boundary line that has a ________ slope,the substance can go from solid to liquid,within a small temperature range,via the application of pressure.
A)positive
B)zero
C)y = 1
D)negative
E)y = 0
A)positive
B)zero
C)y = 1
D)negative
E)y = 0

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
46
On a phase diagram,the critical temperature is ________.
A)the temperature below which a gas cannot be liquefied
B)the temperature above which a gas cannot be liquefied
C)the temperature at which all three states are in equilibrium
D)the temperature required to melt a solid
E)the temperature required to cause sublimation of a solid
A)the temperature below which a gas cannot be liquefied
B)the temperature above which a gas cannot be liquefied
C)the temperature at which all three states are in equilibrium
D)the temperature required to melt a solid
E)the temperature required to cause sublimation of a solid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
47
Of the following,________ is an exothermic process.
A)melting
B)subliming
C)freezing
D)boiling
E)All of the above are exothermic.
A)melting
B)subliming
C)freezing
D)boiling
E)All of the above are exothermic.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
48
Volatility and vapor pressure are ________.
A)inversely proportional to one another
B)directly proportional to one another
C)not related
D)the same thing
E)both independent of temperature
A)inversely proportional to one another
B)directly proportional to one another
C)not related
D)the same thing
E)both independent of temperature

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
49
The substance with the largest heat of vaporization is ________.
A)H2
B)Cl2
C)I2
D)N2
E)O2
A)H2
B)Cl2
C)I2
D)N2
E)O2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
50
The vapor pressure of a liquid ________.
A)increases linearly with increasing temperature
B)increases nonlinearly with increasing temperature
C)decreases linearly with increasing temperature
D)decreases nonlinearly with increasing temperature
E)is totally unrelated to its molecular structure
A)increases linearly with increasing temperature
B)increases nonlinearly with increasing temperature
C)decreases linearly with increasing temperature
D)decreases nonlinearly with increasing temperature
E)is totally unrelated to its molecular structure

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is most likely to exhibit liquid-crystalline behavior?
A)CH3CH2-C(CH3)2-CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CH2CH2-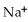
D)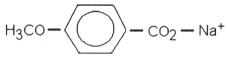
E)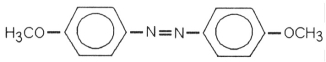
A)CH3CH2-C(CH3)2-CH2CH3
B)CH3CH2CH2CH2CH2CH2CH2CH3
C)CH3CH2CH2CH2CH2-
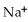
D)

E)
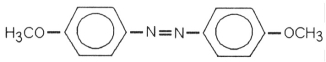

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
52
In general,the vapor pressure of a substance increases as ________ increases.
A)surface tension
B)molecular weight
C)hydrogen bonding
D)viscosity
E)temperature
A)surface tension
B)molecular weight
C)hydrogen bonding
D)viscosity
E)temperature

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
53
A volatile liquid is one that ________.
A)is highly flammable
B)is highly viscous
C)is highly hydrogen-bonded
D)is highly cohesive
E)readily evaporates
A)is highly flammable
B)is highly viscous
C)is highly hydrogen-bonded
D)is highly cohesive
E)readily evaporates

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
54
On a phase diagram,the critical pressure is ________.
A)the pressure required to melt a solid
B)the pressure below which a substance is a solid at all temperatures
C)the pressure above which a substance is a liquid at all temperatures
D)the pressure at which a liquid changes to a gas
E)the pressure required to liquefy a gas at its critical temperature
A)the pressure required to melt a solid
B)the pressure below which a substance is a solid at all temperatures
C)the pressure above which a substance is a liquid at all temperatures
D)the pressure at which a liquid changes to a gas
E)the pressure required to liquefy a gas at its critical temperature

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
55
All of the following are characteristics of liquid crystal behavior except ________.
A)long axial structure
B)carbon-carbon single bonds
C)double bonding
D)ionic configuration
E)polar groups
A)long axial structure
B)carbon-carbon single bonds
C)double bonding
D)ionic configuration
E)polar groups

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
56
The critical temperature and pressure of CS2 are 279 °C and 78 atm,respectively.A supercritical fluid can only exist at a temperature ________ 279 °C and pressure ________ 78 atm.
A)exactly, exactly
B)above, below
C)above, above
D)below, above
E)below, below
A)exactly, exactly
B)above, below
C)above, above
D)below, above
E)below, below

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
57
The predominant intramolecular force in CaBr2 is ________.
A)London-dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding
A)London-dispersion forces
B)ion-dipole forces
C)ionic bonding
D)dipole-dipole forces
E)hydrogen bonding

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
58
Of the following,________ should have the highest critical temperature.
A)CBr4
B)CCl4
C)CF4
D)CH4
E)H2
A)CBr4
B)CCl4
C)CF4
D)CH4
E)H2

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
59
The vapor pressure of any substance at its normal boiling point is ________.
A)1 Pa
B)1 torr
C)1 atm
D)equal to atmospheric pressure
E)equal to the vapor pressure of water
A)1 Pa
B)1 torr
C)1 atm
D)equal to atmospheric pressure
E)equal to the vapor pressure of water

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
60
- 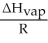 is the slope of a plot of the natural log of the vapor pressure of a substance versus ________.
is the slope of a plot of the natural log of the vapor pressure of a substance versus ________.
A)-1/T
B)-T
C)1/T
D)T
E)2T
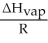 is the slope of a plot of the natural log of the vapor pressure of a substance versus ________.
is the slope of a plot of the natural log of the vapor pressure of a substance versus ________.A)-1/T
B)-T
C)1/T
D)T
E)2T

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
61
According to the phase diagram shown above,what is the normal melting point (°C)?
A)15
B)25
C)35
D)45
E)55
A)15
B)25
C)35
D)45
E)55

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
62
The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated.The slope of the E-F segment corresponds to the heat capacity of the ________.
A)liquid-gas
B)solid-gas
C)gas
D)solid
E)liquid
A)liquid-gas
B)solid-gas
C)gas
D)solid
E)liquid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
63
Molecules with ________ do not generally exhibit liquid-crystalline properties because they lack the rigidity necessary for alignment.
A)both single and double bonds
B)double or triple bonds
C)single, double, and triple bonds
D)only single bonds
E)only single and double bonds
A)both single and double bonds
B)double or triple bonds
C)single, double, and triple bonds
D)only single bonds
E)only single and double bonds

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
64
In the ________ liquid crystalline phase,the component molecules exhibit ________ dimensional ordering.
A)nematic, one
B)smectic A, one
C)nematic, two
D)nematic, three
E)smectic B, one
A)nematic, one
B)smectic A, one
C)nematic, two
D)nematic, three
E)smectic B, one

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
65
The phase diagram of a substance is given above.The region that corresponds to the solid phase is ________.
A)w
B)x
C)y
D)z
E)x and y
A)w
B)x
C)y
D)z
E)x and y

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
66
The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated.The slope of the ________ segment corresponds to the heat capacity of the liquid of the substance.
A)AB
B)BC
C)CD
D)DE
E)EF
A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
67
Based on molecular mass and dipole moment of the five compounds in the table below,which should have the highest boiling point? 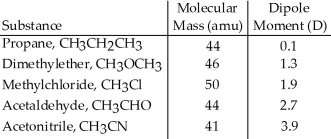
A)CH3CH2CH3
B)CH3OCH3
C)CH3Cl
D)CH3CHO
E)CH3CN

A)CH3CH2CH3
B)CH3OCH3
C)CH3Cl
D)CH3CHO
E)CH3CN

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
68
On the phase diagram shown above,segment ________ corresponds to the conditions of temperature and pressure under which the solid and the gas of the substance are in equilibrium.
A)AB
B)AC
C)AD
D)CD
E)BC
A)AB
B)AC
C)AD
D)CD
E)BC

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
69
On the phase diagram shown above,the coordinates of point B corresponds to the ________.
A)critical temperature and pressure
B)critical pressure
C)critical temperature
D)boiling point
E)triple point
A)critical temperature and pressure
B)critical pressure
C)critical temperature
D)boiling point
E)triple point

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
70
For a given substance that exhibits liquid-crystalline properties,the transition from solid to liquid-crystal state occurs ________.
A)over a range of temperatures between the melting point of the solid and the boiling point of the liquid
B)at the melting point of the solid
C)over a range of temperatures that includes the melting point of the solid
D)at a well-defined temperature above the melting point of the solid
E)at a well-defined temperature below the melting point of the solid
A)over a range of temperatures between the melting point of the solid and the boiling point of the liquid
B)at the melting point of the solid
C)over a range of temperatures that includes the melting point of the solid
D)at a well-defined temperature above the melting point of the solid
E)at a well-defined temperature below the melting point of the solid

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
71
________ liquid crystals are colored because the molecular layers are arranged in slightly twisted planes with respect to one another.
A)smectic B
B)cholesteric
C)smectic A
D)smectic C
E)smectic D
A)smectic B
B)cholesteric
C)smectic A
D)smectic C
E)smectic D

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
72
The phase diagram of a substance is shown above.The area labeled ________ indicates the gas phase for the substance.
A)w
B)x
C)y
D)z
E)y and z
A)w
B)x
C)y
D)z
E)y and z

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
73
On the phase diagram shown above,the coordinates of point ________ correspond to the triple point.
A)A
B)B
C)C
D)D
E)E
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
74
The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated.The slope of the ________ segment corresponds to the heat capacity of the solid.
A)AB
B)BC
C)CD
D)DE
E)EF
A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
75
The phase diagram of a substance is shown above.The area labeled ________ indicates the solid phase for the substance.
A)w
B)x
C)y
D)z
E)y and z
A)w
B)x
C)y
D)z
E)y and z

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
76
There are ________ types of smectic liquid-crystalline phases.
A)6
B)5
C)2
D)3
E)4
A)6
B)5
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
77
The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated.The heat flow into the sample in the segment ________ will yield the value of the ΔHfusion of this substance.
A)AB
B)BC
C)CD
D)DE
E)EF
A)AB
B)BC
C)CD
D)DE
E)EF

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
78
According to the phase diagram shown above,what is the normal boiling point (°C)?
A)10
B)20
C)30
D)40
E)50
A)10
B)20
C)30
D)40
E)50

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
79
The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated.The heat flow into the sample in the segment D-E will yield the value of the ________ of this substance.
A)ΔHsub
B)ΔHvap
C)ΔHmelting
D)ΔHfusion
E)ΔHrxn
A)ΔHsub
B)ΔHvap
C)ΔHmelting
D)ΔHfusion
E)ΔHrxn

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
80
What are the common types of smectic liquid-crystalline phases?
A)A, C, and D
B)A only
C)A and C
D)A and D
E)C and D
A)A, C, and D
B)A only
C)A and C
D)A and D
E)C and D

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck


