Deck 17: Additional Aspects of Aqueous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/116
Play
Full screen (f)
Deck 17: Additional Aspects of Aqueous Equilibria
1
Which of the following could be added to a solution of NaF to prepare a buffer?
A)HBr
B)NaOH
C)LiC2H3O2
D)KF
E)NH3
A)HBr
B)NaOH
C)LiC2H3O2
D)KF
E)NH3
HBr
2
Which of the following could be added to a solution of sodium acetate to produce a buffer?
A)acetic acid only
B)acetic acid or hydrochloric acid
C)hydrochloric acid only
D)potassium acetate only
E)sodium chloride or potassium acetate
A)acetic acid only
B)acetic acid or hydrochloric acid
C)hydrochloric acid only
D)potassium acetate only
E)sodium chloride or potassium acetate
acetic acid or hydrochloric acid
3
Which of the following could be added to a solution of HC2H3O2 to prepare a buffer?
A)HBr
B)HNO3
C)KOH
D)more HC2H3O2
E)None of the above can be added to an acetic acid solution to prepare a buffer.
A)HBr
B)HNO3
C)KOH
D)more HC2H3O2
E)None of the above can be added to an acetic acid solution to prepare a buffer.
KOH
4
The Henderson-Hasselbalch equation is ________.
A)[H+] = Ka +![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_c997_9a0a_8fa4c2398b13_TB1194_11.jpg)
B)pH = pKa - log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_c998_9a0a_1b43014d9f2e_TB1194_11.jpg)
C)pH = pKa + log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_c999_9a0a_7973f922a195_TB1194_11.jpg)
D)pH = pKa + log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_f0aa_9a0a_4d2381041ab6_TB1194_11.jpg)
E)pH = log![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_f0ab_9a0a_e1acd2d4bb9e_TB1194_11.jpg)
A)[H+] = Ka +
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_c997_9a0a_8fa4c2398b13_TB1194_11.jpg)
B)pH = pKa - log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_c998_9a0a_1b43014d9f2e_TB1194_11.jpg)
C)pH = pKa + log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_c999_9a0a_7973f922a195_TB1194_11.jpg)
D)pH = pKa + log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_f0aa_9a0a_4d2381041ab6_TB1194_11.jpg)
E)pH = log
![<strong>The Henderson-Hasselbalch equation is ________.</strong> A)[H<sup>+</sup>] = K<sub>a</sub> + B)pH = pK<sub>a</sub> - log C)pH = pK<sub>a</sub> + log D)pH = pK<sub>a</sub> + log E)pH = log](https://storage.examlex.com/TB1194/11ea7e7c_6162_f0ab_9a0a_e1acd2d4bb9e_TB1194_11.jpg)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
5
The addition of HCl and ________ to water produces a buffer solution.
A)HC6H5O
B)C2H5NH2
C)KOH
D)KCl
E)none of the above
A)HC6H5O
B)C2H5NH2
C)KOH
D)KCl
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following pairs cannot be mixed together to form a buffer solution?
A)C5H5N, C5H5NHCl
B)HC2H3O2, NaOH (C2H3O2- = acetate)
C)KOH, HI
D)NH2CH3, HCl
E)NaClO, HNO3
A)C5H5N, C5H5NHCl
B)HC2H3O2, NaOH (C2H3O2- = acetate)
C)KOH, HI
D)NH2CH3, HCl
E)NaClO, HNO3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
7
A solution containing which one of the following pairs of substances will be a buffer solution?
A)KI, HI
B)AgBr, HBr
C)CuCl, HCl
D)CsI, HI
E)none of the above
A)KI, HI
B)AgBr, HBr
C)CuCl, HCl
D)CsI, HI
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following pairs cannot be mixed together to form a buffer solution?
A)HONH2, HONH3Cl
B)NaCl, HCl
C)RbOH, HF
D)KOH, HNO2
E)H2SO3, KHSO3
A)HONH2, HONH3Cl
B)NaCl, HCl
C)RbOH, HF
D)KOH, HNO2
E)H2SO3, KHSO3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
9
Which solution has the greatest buffering capacity?
A)0.335M HC2H3O2 and 0.497 M NaC2H3O2
B)0.520 M HC2H3O2 and 0.116 M NaC2H3O2
C)0.820 M HC2H3O2 and 0.715 M NaC2H3O2
D)0.120 M HC2H3O2 and 0.115 M NaC2H3O2
A)0.335M HC2H3O2 and 0.497 M NaC2H3O2
B)0.520 M HC2H3O2 and 0.116 M NaC2H3O2
C)0.820 M HC2H3O2 and 0.715 M NaC2H3O2
D)0.120 M HC2H3O2 and 0.115 M NaC2H3O2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
10
What is the primary buffer system that controls the pH of the blood?
A)carbonate, bicarbonate
B)carbon dioxide, carbonate
C)carbonic acid, bicarbonate
D)carbonic acid, carbon dioxide
E)carbonate, carbonic acid
A)carbonate, bicarbonate
B)carbon dioxide, carbonate
C)carbonic acid, bicarbonate
D)carbonic acid, carbon dioxide
E)carbonate, carbonic acid

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
11
In a solution,when the concentrations of a weak acid and its conjugate base are equal,________.
A)the system is not at equilibrium
B)the buffering capacity is significantly decreased
C)the -log of the [H+] and the -log of the Ka are equal
D)All of the above are true.
A)the system is not at equilibrium
B)the buffering capacity is significantly decreased
C)the -log of the [H+] and the -log of the Ka are equal
D)All of the above are true.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
12
The addition of HCl and ________ to water produces a buffer solution.
A)NH3
B)HC6H5O
C)KOH
D)KNO3
E)HNO3
A)NH3
B)HC6H5O
C)KOH
D)KNO3
E)HNO3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
13
Which solution has the greatest buffering capacity?
A)0.335 M NH3 and 0.100 M NH4Cl
B)0.085 M NH3 and 0.090 M NH4Cl
C)0.540 M NH3 and 0.550 M NH4Cl
D)0.200 M NH3 and 0.565 M NH4Cl
E)They are all buffer solutions and would all have the same capacity.
A)0.335 M NH3 and 0.100 M NH4Cl
B)0.085 M NH3 and 0.090 M NH4Cl
C)0.540 M NH3 and 0.550 M NH4Cl
D)0.200 M NH3 and 0.565 M NH4Cl
E)They are all buffer solutions and would all have the same capacity.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following could be added to a solution of acetic acid to prepare a buffer?
A)sodium acetate only
B)sodium acetate or sodium hydroxide
C)nitric acid only
D)hydrofluoric acid or nitric acid
E)sodium hydroxide only
A)sodium acetate only
B)sodium acetate or sodium hydroxide
C)nitric acid only
D)hydrofluoric acid or nitric acid
E)sodium hydroxide only

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
15
What change will be caused by addition of a small amount of HCl to a solution containing fluoride ions and hydrogen fluoride?
A)The concentration of hydronium ions will increase significantly.
B)The concentration of fluoride ions will increase as will the concentration of hydronium ions.
C)The concentration of hydrogen fluoride will decrease and the concentration of fluoride ions will increase.
D)The concentration of fluoride ion will decrease and the concentration of hydrogen fluoride will increase.
E)The fluoride ions will precipitate out of solution as its acid salt.
A)The concentration of hydronium ions will increase significantly.
B)The concentration of fluoride ions will increase as will the concentration of hydronium ions.
C)The concentration of hydrogen fluoride will decrease and the concentration of fluoride ions will increase.
D)The concentration of fluoride ion will decrease and the concentration of hydrogen fluoride will increase.
E)The fluoride ions will precipitate out of solution as its acid salt.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
16
The addition of HF and ________ to water produces a buffer solution.
A)HBr
B)KNO3
C)KOH
D)NaCl
E)NaBr
A)HBr
B)KNO3
C)KOH
D)NaCl
E)NaBr

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
17
The ________ and ________ are the principal organs that regulate the pH of the carbonic acid-bicarbonate buffer system in the blood.
A)kidneys, liver
B)lungs, skin
C)lungs, kidneys
D)brain stem, heart
E)spleen, liver
A)kidneys, liver
B)lungs, skin
C)lungs, kidneys
D)brain stem, heart
E)spleen, liver

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following pairs cannot be mixed together to form a buffer solution?
A)NH3, NH4Cl
B)NaC2H3O2, HCl (C2H3O2- = acetate)
C)RbOH, HBr
D)KOH, HF
E)H3PO4, KH2PO4
A)NH3, NH4Cl
B)NaC2H3O2, HCl (C2H3O2- = acetate)
C)RbOH, HBr
D)KOH, HF
E)H3PO4, KH2PO4

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
19
The addition of KOH and ________ to water produces a buffer solution.
A)HI
B)NH3
C)KF
D)LiC2H3O2
E)none of the above
A)HI
B)NH3
C)KF
D)LiC2H3O2
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
20
A solution containing which one of the following pairs of substances will be a buffer solution?
A)NaI, HI
B)KBr, HBr
C)RbCl, HCl
D)CsF, HF
E)none of the above
A)NaI, HI
B)KBr, HBr
C)RbCl, HCl
D)CsF, HF
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
21
The pH of a solution prepared by dissolving 0.550 mol of solid methylamine hydrochloride (CH3NH3Cl)in 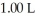 of
of 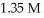 methylamine (CH3NH2)is ________.The Kb for methylamine is
methylamine (CH3NH2)is ________.The Kb for methylamine is 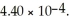 (Assume the final volume is 1.00 L.)
(Assume the final volume is 1.00 L.)
A)11.03
B)2.97
C)3.75
D)10.64
E)10.25
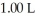 of
of 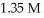 methylamine (CH3NH2)is ________.The Kb for methylamine is
methylamine (CH3NH2)is ________.The Kb for methylamine is 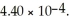 (Assume the final volume is 1.00 L.)
(Assume the final volume is 1.00 L.)A)11.03
B)2.97
C)3.75
D)10.64
E)10.25

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
22
A 25.0 mL sample of 0.723 M HClO4 is titrated with a 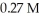 KOH solution.The H3O+ concentration after the addition of
KOH solution.The H3O+ concentration after the addition of 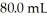 of KOH is ________ M.
of KOH is ________ M.
A)0.4
B)1 × 10-7
C)0.7
D)3 × 10-13
E)4 × 10-2
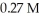 KOH solution.The H3O+ concentration after the addition of
KOH solution.The H3O+ concentration after the addition of 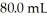 of KOH is ________ M.
of KOH is ________ M.A)0.4
B)1 × 10-7
C)0.7
D)3 × 10-13
E)4 × 10-2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
23
A 25.0 mL sample of a solution of an unknown compound is titrated with a 0.115 M NaOH solution.The titration curve above was obtained.The unknown compound is ________.
A)a strong acid
B)a strong base
C)a weak acid
D)a weak base
E)neither an acid nor a base
A)a strong acid
B)a strong base
C)a weak acid
D)a weak base
E)neither an acid nor a base

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
24
Which compound listed below has the greatest molar solubility in water?
A)CdCO3
B)Cd(OH)2
C)AgI
D)CaF2
E)ZnCO3
A)CdCO3
B)Cd(OH)2
C)AgI
D)CaF2
E)ZnCO3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following is not amphoteric?
A)Al(OH)3
B)Ca(OH)2
C)Cr(OH)3
D)Zn(OH)2
E)Sn(OH)2
A)Al(OH)3
B)Ca(OH)2
C)Cr(OH)3
D)Zn(OH)2
E)Sn(OH)2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
26
Human blood is considered to be ________.
A)neutral
B)very basic
C)slightly basic
D)slightly acidic
E)very acidic
A)neutral
B)very basic
C)slightly basic
D)slightly acidic
E)very acidic

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
27
The Ka of benzoic acid is 6.30 × 10-5.The pH of a buffer prepared by combining 50.0 mL of 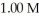 potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
A)1.705
B)0.851
C)3.406
D)4.201
E)2.383
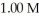 potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.A)1.705
B)0.851
C)3.406
D)4.201
E)2.383

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
28
Which compound listed below has the smallest molar solubility in water?
A)ZnCO3
B)Cd(OH)2
C)CdCO3
D)AgI
E)CaF2
A)ZnCO3
B)Cd(OH)2
C)CdCO3
D)AgI
E)CaF2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
29
A result of the common-ion effect is ________.
A)that some ions, such as Na+ (aq), frequently appear in solutions but do not participate in solubility equilibria
B)that common ions, such as Na+ (aq), don't affect equilibrium constants
C)that the selective precipitation of a metal ion, such as Ag+, is promoted by the addition of an appropriate counterion (X-)that produces a compound (AgX)with a very low solubility
D)that ions such as K+ and Na+ are common ions, so that their values in equilibrium constant expressions are always 1.00
E)that common ions precipitate all counter-ions
A)that some ions, such as Na+ (aq), frequently appear in solutions but do not participate in solubility equilibria
B)that common ions, such as Na+ (aq), don't affect equilibrium constants
C)that the selective precipitation of a metal ion, such as Ag+, is promoted by the addition of an appropriate counterion (X-)that produces a compound (AgX)with a very low solubility
D)that ions such as K+ and Na+ are common ions, so that their values in equilibrium constant expressions are always 1.00
E)that common ions precipitate all counter-ions

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
30
For which salt should the aqueous solubility be most sensitive to pH?
A)MgCl2
B)Mg(NO3)2
C)MgF2
D)MgBr2
E)MgI2
A)MgCl2
B)Mg(NO3)2
C)MgF2
D)MgBr2
E)MgI2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following is amphoteric?
A)H2SO4
B)H2O2
C)CO2
D)H2O
E)NaOH
A)H2SO4
B)H2O2
C)CO2
D)H2O
E)NaOH

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
32
A 50.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution.The titration curve above was obtained.The concentration of the monoprotic acid is about ________ mol/L.
A)0.120
B)25.0
C)0.240
D)0.0600
E)0.100
A)0.120
B)25.0
C)0.240
D)0.0600
E)0.100

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
33
The molar solubility of ________ is not affected by the pH of the solution.
A)Na3PO4
B)NaF
C)KNO3
D)AlCl3
E)MnS
A)Na3PO4
B)NaF
C)KNO3
D)AlCl3
E)MnS

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
34
Why does fluoride treatment render teeth more resistant to decay?
A)Fluoride kills the bacteria in the mouth that make the acids that decay teeth.
B)Fluoride stimulates production of tooth enamel to replace that lost to decay.
C)Fluoride reduces saliva production, keeping teeth drier and thus reducing decay.
D)Fluoride converts hydroxyapatite to fluoroapatite that is less reactive with acids.
E)Fluoride dissolves plaque, reducing its decaying contact with teeth.
A)Fluoride kills the bacteria in the mouth that make the acids that decay teeth.
B)Fluoride stimulates production of tooth enamel to replace that lost to decay.
C)Fluoride reduces saliva production, keeping teeth drier and thus reducing decay.
D)Fluoride converts hydroxyapatite to fluoroapatite that is less reactive with acids.
E)Fluoride dissolves plaque, reducing its decaying contact with teeth.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
35
A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution.The titration curve above was obtained.Which of the following indicators would be best for this titration?
A)methyl red
B)bromthymol blue
C)thymol blue
D)phenolpthalein
E)bromocresol purple
A)methyl red
B)bromthymol blue
C)thymol blue
D)phenolpthalein
E)bromocresol purple

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the pH of a solution prepared by dissolving 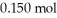 of benzoic acid and
of benzoic acid and 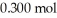 of sodium benzoate in water sufficient to yield
of sodium benzoate in water sufficient to yield 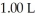 of solution.The Ka of benzoic acid is
of solution.The Ka of benzoic acid is 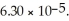
A)2.516
B)3.892
C)4.502
D)10.158
E)4.195
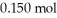 of benzoic acid and
of benzoic acid and 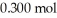 of sodium benzoate in water sufficient to yield
of sodium benzoate in water sufficient to yield 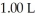 of solution.The Ka of benzoic acid is
of solution.The Ka of benzoic acid is 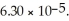
A)2.516
B)3.892
C)4.502
D)10.158
E)4.195

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the pH of a solution prepared by dissolving 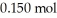 of acetic acid and
of acetic acid and 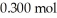 of sodium acetate in water sufficient to yield
of sodium acetate in water sufficient to yield 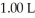 of solution.The Ka of acetic acid is
of solution.The Ka of acetic acid is 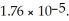
A)2.516
B)3.892
C)4.502
D)10.158
E)5.056
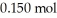 of acetic acid and
of acetic acid and 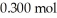 of sodium acetate in water sufficient to yield
of sodium acetate in water sufficient to yield 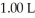 of solution.The Ka of acetic acid is
of solution.The Ka of acetic acid is 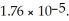
A)2.516
B)3.892
C)4.502
D)10.158
E)5.056

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
38
The pH of a solution prepared by dissolving 0.350 mol of acid in 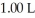 of
of  of conjugate base is ________.The Kb for the conjugate base is
of conjugate base is ________.The Kb for the conjugate base is 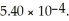 (Assume the final volume is 1.00 L.)
(Assume the final volume is 1.00 L.)
A)11.23
B)1.66
C)11.14
D)2.77
E)none of the above
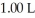 of
of  of conjugate base is ________.The Kb for the conjugate base is
of conjugate base is ________.The Kb for the conjugate base is 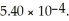 (Assume the final volume is 1.00 L.)
(Assume the final volume is 1.00 L.)A)11.23
B)1.66
C)11.14
D)2.77
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
39
In which one of the following solutions is silver chloride the most soluble?
A)0.200 M HCl
B)0.750 M LiNO3
C)0.0150 M NH3
D)0.185 M KCl
E)pure H2O
A)0.200 M HCl
B)0.750 M LiNO3
C)0.0150 M NH3
D)0.185 M KCl
E)pure H2O

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
40
Decreasing the pH of blood will cause hemoglobin to release ________.
A)CO2
B)N2
C)H2
D)O2
E)Fe
A)CO2
B)N2
C)H2
D)O2
E)Fe

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
41
The pH of a solution prepared by mixing 50.0 mL of 0.125 M NaOH and 40.0 mL of 0.125 M HNO3 is ________.
A)13.29
B)7.00
C)8.11
D)11.00
E)12.14
A)13.29
B)7.00
C)8.11
D)11.00
E)12.14

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
42
The Ksp for Cu(OH)2 is 4.8 × 10-20.Determine the molar solubility of Cu(OH)2 in a buffer solution with a pH of 10.1.
A)6.0 × 10-10
B)7.6
C)3.0 × 10-12
D)2.2 × 10-10
E)3.8 × 10-16
A)6.0 × 10-10
B)7.6
C)3.0 × 10-12
D)2.2 × 10-10
E)3.8 × 10-16

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
43
The solubility of lead (II)chloride (PbCl2)is 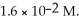 What is the Ksp of PbCl2?
What is the Ksp of PbCl2?
A)5.0 × 10-4
B)4.1 × 10-6
C)3.1 × 10-7
D)1.6 × 10-5
E)1.6 × 10-2
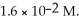 What is the Ksp of PbCl2?
What is the Ksp of PbCl2?A)5.0 × 10-4
B)4.1 × 10-6
C)3.1 × 10-7
D)1.6 × 10-5
E)1.6 × 10-2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
44
What is the solubility (in M)of PbCl2 in a 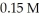 solution of HCl? The Ksp of PbCl2 is
solution of HCl? The Ksp of PbCl2 is 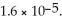
A)2.0 × 10-3
B)1.1 × 10-4
C)1.8 × 10-4
D)7.1 × 10-4
E)1.6 × 10-5
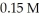 solution of HCl? The Ksp of PbCl2 is
solution of HCl? The Ksp of PbCl2 is 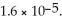
A)2.0 × 10-3
B)1.1 × 10-4
C)1.8 × 10-4
D)7.1 × 10-4
E)1.6 × 10-5

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
45
The concentration of iodide ions in a saturated solution of silver iodide is ________ M.The solubility product constant of AgI is 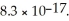
A)3.8 × 10-11
B)3.0 × 10-10
C)9.1 × 10-9
D)3.5 × 10-9
E)1.4 × 10-8
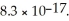
A)3.8 × 10-11
B)3.0 × 10-10
C)9.1 × 10-9
D)3.5 × 10-9
E)1.4 × 10-8

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the pH of a solution that is 0.278 M in sodium formate ( 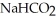 )and
)and 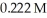 in formic acid
in formic acid 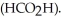 The
The 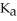 of formic acid is 1.77 ×
of formic acid is 1.77 × 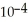 .
.
A)3.843
B)3.647
C)13.90
D)10.16
E)4.954
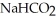 )and
)and 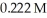 in formic acid
in formic acid 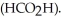 The
The 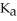 of formic acid is 1.77 ×
of formic acid is 1.77 × 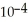 .
.A)3.843
B)3.647
C)13.90
D)10.16
E)4.954

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
47
The concentration of iodide ions in a saturated solution of lead (II)iodide is ________ M.The solubility product constant of PbI2 is 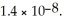
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-3
D)3.5 × 10-9
E)1.4 × 10-8
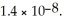
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-3
D)3.5 × 10-9
E)1.4 × 10-8

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the percent ionization of formic acid ( 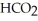 H)in a solution that is 0.322 M in formic acid and 0.178 M in sodium formate (
H)in a solution that is 0.322 M in formic acid and 0.178 M in sodium formate ( 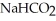 ).The
).The 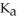 of formic acid is 1.77 ×
of formic acid is 1.77 × 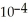 .
.
A)35.6
B)0.1011
C)10.8
D)1.03 × 10-3
E)3.488
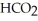 H)in a solution that is 0.322 M in formic acid and 0.178 M in sodium formate (
H)in a solution that is 0.322 M in formic acid and 0.178 M in sodium formate ( 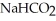 ).The
).The 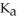 of formic acid is 1.77 ×
of formic acid is 1.77 × 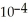 .
.A)35.6
B)0.1011
C)10.8
D)1.03 × 10-3
E)3.488

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the maximum concentration (in M)of silver ions (Ag+)in a solution that contains 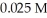 of CO32-.The Ksp of Ag2CO3 is
of CO32-.The Ksp of Ag2CO3 is 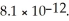
A)1.8 × 10-5
B)1.4 × 10-6
C)2.8 × 10-6
D)3.2 × 10-10
E)8.1 × 10-12
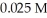 of CO32-.The Ksp of Ag2CO3 is
of CO32-.The Ksp of Ag2CO3 is 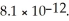
A)1.8 × 10-5
B)1.4 × 10-6
C)2.8 × 10-6
D)3.2 × 10-10
E)8.1 × 10-12

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
50
What is the percent ionization of nitrous acid in a solution that is 0.222 M in nitrous acid (HNO2)and 0.278 M in potassium nitrite (KNO2)? The acid dissociation constant of nitrous acid is 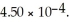
A)55.6
B)15.5
C)2.78 ×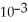
D)3.448
E)0.162
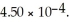
A)55.6
B)15.5
C)2.78 ×
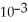
D)3.448
E)0.162

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
51
A 50.0 mL sample of an aqueous H2SO4 solution is titrated with a 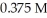 NaOH solution.The equivalence point is reached with
NaOH solution.The equivalence point is reached with 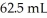 of the base.The concentration of H2SO4 is ________ M.
of the base.The concentration of H2SO4 is ________ M.
A)0.234
B)0.469
C)0.150
D)0.300
E)0.938
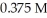 NaOH solution.The equivalence point is reached with
NaOH solution.The equivalence point is reached with 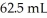 of the base.The concentration of H2SO4 is ________ M.
of the base.The concentration of H2SO4 is ________ M.A)0.234
B)0.469
C)0.150
D)0.300
E)0.938

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
52
The pH of a solution prepared by mixing 50.0 mL of 0.125 M KOH and 50.0 mL of 0.125 M HCl is ________.
A)6.29
B)7.00
C)8.11
D)5.78
E)0.00
A)6.29
B)7.00
C)8.11
D)5.78
E)0.00

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
53
The concentration of fluoride ions in a saturated solution of barium fluoride is ________ M.The solubility product constant of BaF2 is 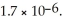
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-2
D)7.5 × 10-3
E)1.4 × 10-4
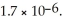
A)3.8 × 10-4
B)3.0 × 10-3
C)1.5 × 10-2
D)7.5 × 10-3
E)1.4 × 10-4

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
54
What is the percent ionization of nitrous acid in a solution that is 0.189 M in nitrous acid? The acid dissociation constant of nitrous acid is 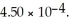
A)0.0450
B)8.51 × 10-5
C)0.594
D)4.20
E)4.88
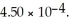
A)0.0450
B)8.51 × 10-5
C)0.594
D)4.20
E)4.88

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the percent ionization of formic acid ( 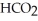 H)in a solution that is 0.152 M in formic acid.The
H)in a solution that is 0.152 M in formic acid.The 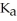 of formic acid is 1.77 ×
of formic acid is 1.77 × 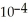 .
.
A)2.74 ×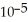
B)0.0180
C)3.44
D)0.581
E)8.44
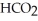 H)in a solution that is 0.152 M in formic acid.The
H)in a solution that is 0.152 M in formic acid.The 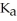 of formic acid is 1.77 ×
of formic acid is 1.77 × 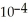 .
.A)2.74 ×
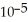
B)0.0180
C)3.44
D)0.581
E)8.44

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
56
The solubility of manganese (II)hydroxide (Mn(OH)2)is 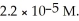 What is the Ksp of Mn(OH)2?
What is the Ksp of Mn(OH)2?
A)1.1 × 10-14
B)4.3 × 10-14
C)2.1 × 10-14
D)4.8 × 10-10
E)2.2 × 10-5
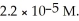 What is the Ksp of Mn(OH)2?
What is the Ksp of Mn(OH)2?A)1.1 × 10-14
B)4.3 × 10-14
C)2.1 × 10-14
D)4.8 × 10-10
E)2.2 × 10-5

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
57
The pH of a solution prepared by mixing 40.0 mL of 0.125 M Mg(OH)2 and 150.0 mL of 0.125 M HCl is ________.
A)6.29
B)4.11
C)1.14
D)5.78
E)1.34
A)6.29
B)4.11
C)1.14
D)5.78
E)1.34

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
58
Determine the Ksp for magnesium hydroxide (Mg(OH)2)where the solubility of Mg(OH)2 is 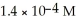 .
.
A)2.7 × 10-12
B)1.1 × 10-11
C)2.0 × 10-8
D)3.9 × 10-8
E)1.4 × 10-4
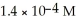 .
.A)2.7 × 10-12
B)1.1 × 10-11
C)2.0 × 10-8
D)3.9 × 10-8
E)1.4 × 10-4

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the pH of a solution that is 0.322 M in nitrous acid ( 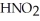 )and 0.178 M in potassium nitrite (KNO2).The acid dissociation constant of nitrous acid is 4.50 ×
)and 0.178 M in potassium nitrite (KNO2).The acid dissociation constant of nitrous acid is 4.50 × 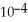 .
.
A)3.093
B)3.607
C)14.26
D)10.91
E)4.589
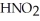 )and 0.178 M in potassium nitrite (KNO2).The acid dissociation constant of nitrous acid is 4.50 ×
)and 0.178 M in potassium nitrite (KNO2).The acid dissociation constant of nitrous acid is 4.50 × 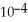 .
.A)3.093
B)3.607
C)14.26
D)10.91
E)4.589

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the maximum concentration (in M)of calcium ions (Ca2+)in a solution that contains 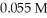 of CO32-.The Ksp of CaCO3 is
of CO32-.The Ksp of CaCO3 is 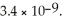
A)5.8 × 10-5
B)6.8 × 10-9
C)3.4 × 10-9
D)6.2 × 10-8
E)1.9 × 10-10
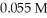 of CO32-.The Ksp of CaCO3 is
of CO32-.The Ksp of CaCO3 is 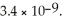
A)5.8 × 10-5
B)6.8 × 10-9
C)3.4 × 10-9
D)6.2 × 10-8
E)1.9 × 10-10

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
61
What is the pH of a buffer solution that is 0.266 M in lactic acid and 0.111 M in sodium lactate? The 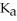 of lactic acid is 1.4 ×
of lactic acid is 1.4 × 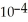 .
.
A)14.38
B)10.53
C)5.38
D)3.47
E)4.23
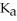 of lactic acid is 1.4 ×
of lactic acid is 1.4 × 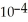 .
.A)14.38
B)10.53
C)5.38
D)3.47
E)4.23

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
62
Which solution would have the greatest buffering capacity?
A)0.574 M HF and 0.312 M NaF
B)0.287 M HF and 0.156 M NaF
C)0.189 M HF and 0.103 M NaF
D)1.15 M HF and 0.624 M NaF
E)They are all buffer solutions and would all have the same capacity.
A)0.574 M HF and 0.312 M NaF
B)0.287 M HF and 0.156 M NaF
C)0.189 M HF and 0.103 M NaF
D)1.15 M HF and 0.624 M NaF
E)They are all buffer solutions and would all have the same capacity.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
63
The 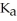 of some weak acid HA is 1.76 ×
of some weak acid HA is 1.76 × 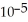 .The pH of a buffer prepared by combining 15.0 mL of
.The pH of a buffer prepared by combining 15.0 mL of 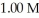 A- and 50.0 mL of 1.00 M HA is ________.
A- and 50.0 mL of 1.00 M HA is ________.
A)1.705
B)4.232
C)0.851
D)2.383
E)3.406
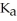 of some weak acid HA is 1.76 ×
of some weak acid HA is 1.76 × 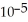 .The pH of a buffer prepared by combining 15.0 mL of
.The pH of a buffer prepared by combining 15.0 mL of 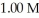 A- and 50.0 mL of 1.00 M HA is ________.
A- and 50.0 mL of 1.00 M HA is ________.A)1.705
B)4.232
C)0.851
D)2.383
E)3.406

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
64
A solution is prepared by dissolving 0.23 mol of hypochlorous acid and 0.27 mol of sodium hypochlorite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 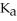 of hypochlorous acid is 1.36 × 10-3.
of hypochlorous acid is 1.36 × 10-3.
A) O
O
B)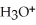
C)hypochlorite ion
D)hypochlorous acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.
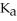 of hypochlorous acid is 1.36 × 10-3.
of hypochlorous acid is 1.36 × 10-3.A)
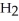 O
OB)
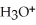
C)hypochlorite ion
D)hypochlorous acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
65
The 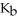 of ammonia is 1.76 ×
of ammonia is 1.76 × 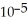 .What is the pH of a buffer which is prepared by combining 50.0 mL of 1.00 M ammonia and 45.0 mL of 1.00 M ammonium nitrate?
.What is the pH of a buffer which is prepared by combining 50.0 mL of 1.00 M ammonia and 45.0 mL of 1.00 M ammonium nitrate?
A)9.372
B)4.632
C)4.742
D)9.291
E)none of the above
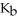 of ammonia is 1.76 ×
of ammonia is 1.76 × 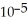 .What is the pH of a buffer which is prepared by combining 50.0 mL of 1.00 M ammonia and 45.0 mL of 1.00 M ammonium nitrate?
.What is the pH of a buffer which is prepared by combining 50.0 mL of 1.00 M ammonia and 45.0 mL of 1.00 M ammonium nitrate?A)9.372
B)4.632
C)4.742
D)9.291
E)none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
66
A buffer solution with a pH of 4.31 is prepared with 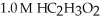 and
and 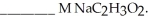 The Ka of H
The Ka of H 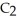
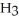
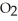 is
is 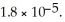
A)0.37
B)0.74
C)4.2 × 10-6
D)8.8 × 10-10
E)0.18
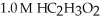 and
and 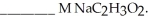 The Ka of H
The Ka of H 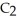
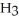
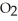 is
is 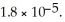
A)0.37
B)0.74
C)4.2 × 10-6
D)8.8 × 10-10
E)0.18

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
67
0.78 M Na 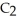
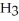
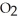 and
and 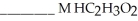 are required to prepare a buffer solution with a pH of 4.40 .The
are required to prepare a buffer solution with a pH of 4.40 .The 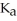 of H
of H 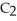
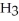
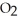 is
is 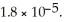
A)3.5
B)4.1 × 104
C)1.7
D)0.86
E)0.35
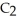
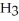
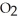 and
and 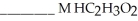 are required to prepare a buffer solution with a pH of 4.40 .The
are required to prepare a buffer solution with a pH of 4.40 .The 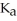 of H
of H 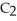
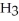
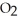 is
is 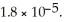
A)3.5
B)4.1 × 104
C)1.7
D)0.86
E)0.35

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
68
What is the pH of a solution which is prepared by dissolving 0.850 mol of N 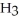 and
and 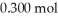 of
of 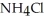 in water sufficient to yield 1.00 L of solution? The
in water sufficient to yield 1.00 L of solution? The 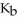 of ammonia is
of ammonia is 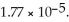
A)9.700
B)5.204
C)8.781
D)8.796
E)4.300
 and
and 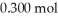 of
of 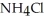 in water sufficient to yield 1.00 L of solution? The
in water sufficient to yield 1.00 L of solution? The 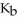 of ammonia is
of ammonia is 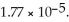
A)9.700
B)5.204
C)8.781
D)8.796
E)4.300

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
69
Consider a solution containing 0.100 M fluoride ions and 0.126 M hydrogen fluoride.The concentration of fluoride ions after the addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution is ________ M.
A)0.0735
B)0.0762
C)0.0980
D)0.0709
E)0.00253
A)0.0735
B)0.0762
C)0.0980
D)0.0709
E)0.00253

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
70
A buffer solution contains 0.100 M fluoride ions and 0.126 M hydrogen fluoride.What is the concentration (M)of hydrogen fluoride after addition of 9.00 mL of 0.0100 M HCl to 25.0 mL of this solution?
A)0.0900
B)0.122
C)0.130
D)0.0953
E)0.00976
A)0.0900
B)0.122
C)0.130
D)0.0953
E)0.00976

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
71
Calculate the pH of a solution prepared by dissolving 0.270 mol of weak acid HA and 0.260 mol of its conjugate base in water sufficient to yield 1.00 L of solution.The 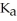 of HA is
of HA is 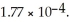
A)2.099
B)3.736
C)10.264
D)3.952
E)2.307
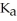 of HA is
of HA is 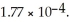
A)2.099
B)3.736
C)10.264
D)3.952
E)2.307

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
72
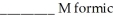 acid and
acid and 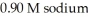 formate are required to prepare a buffer solution with a pH of 4.78 .The
formate are required to prepare a buffer solution with a pH of 4.78 .The 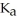 of formic acid is
of formic acid is 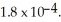
A)0.17
B)0.083
C)3.3 × 103
D)0.041
E)9.8

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
73
A 25.0 mL sample of 0.150 M acetic acid is titrated with a 0.150 M NaOH solution.What is the pH at the equivalence point? The 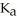 of acetic acid is 4.50 × 10-4.
of acetic acid is 4.50 × 10-4.
A)11.74
B)9.26
C)4.74
D)7.00
E)8.81
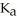 of acetic acid is 4.50 × 10-4.
of acetic acid is 4.50 × 10-4.A)11.74
B)9.26
C)4.74
D)7.00
E)8.81

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
74
What is the pH of a solution that contains 0.800 M weak acid ( 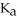 = 1.76 ×
= 1.76 × 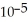 )and 0.172 M of its conjugate base?
)and 0.172 M of its conjugate base?
A)8.578
B)5.422
C)8.370
D)4.087
E)9.913
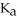 = 1.76 ×
= 1.76 × 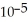 )and 0.172 M of its conjugate base?
)and 0.172 M of its conjugate base?A)8.578
B)5.422
C)8.370
D)4.087
E)9.913

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
75
How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 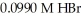 to the equivalence point?
to the equivalence point?
A)29.5
B)0.332
C)4.57
D)0.208
E)21.2
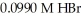 to the equivalence point?
to the equivalence point?A)29.5
B)0.332
C)4.57
D)0.208
E)21.2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
76
The addition of hydrofluoric acid and ________ to water produces a buffer solution.
A)NaF
B)HF
C)NaN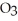
D)NaBr
E)KI
A)NaF
B)HF
C)NaN
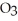
D)NaBr
E)KI

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
77
A solution is prepared by dissolving 0.23 mol of benzoic acid and 0.27 mol of sodium benzoate in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of NaOH to this buffer solution causes the pH to increase slightly.The pH does not increase drastically because the NaOH reacts with the ________ present in the buffer solution.The 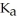 of benzoic acid is 6.3 × 10-5.
of benzoic acid is 6.3 × 10-5.
A)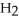 O
O
B)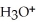
C)benzoate
D)benzoic acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.
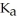 of benzoic acid is 6.3 × 10-5.
of benzoic acid is 6.3 × 10-5.A)
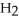 O
OB)
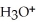
C)benzoate
D)benzoic acid
E)This is a buffer solution: the pH does not change upon addition of acid or base.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
78
What is the pH of a buffer solution that is 0.172 M in hypochlorous acid (HClO)and 0.131 M in sodium hypochlorite? The 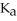 of hypochlorous acid is 3.8 ×
of hypochlorous acid is 3.8 × 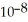 .
.
A)14.12
B)6.70
C)9.07
D)7.54
E)7.30
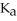 of hypochlorous acid is 3.8 ×
of hypochlorous acid is 3.8 × 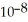 .
.A)14.12
B)6.70
C)9.07
D)7.54
E)7.30

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the pH of a solution prepared by dissolving 0.250 mol of benzoic acid ( 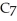
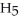
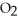 H)and
H)and 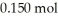 of sodium benzoate (Na
of sodium benzoate (Na 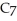
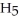
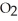 )in water sufficient to yield 1.00 L of solution.The
)in water sufficient to yield 1.00 L of solution.The 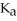 of benzoic acid is
of benzoic acid is 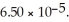
A)4.409
B)3.965
C)10.035
D)9.591
E)5.190
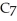
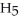
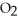 H)and
H)and 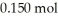 of sodium benzoate (Na
of sodium benzoate (Na 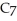
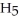
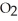 )in water sufficient to yield 1.00 L of solution.The
)in water sufficient to yield 1.00 L of solution.The 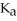 of benzoic acid is
of benzoic acid is 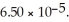
A)4.409
B)3.965
C)10.035
D)9.591
E)5.190

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
80
A buffer solution with a pH of 4.63 is prepared with 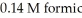 acid and
acid and 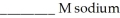 formate. The
formate. The 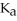 of formic acid is
of formic acid is 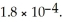
A)1.1
B)2.1
C)5.4 × 10-6
D)3.0 × 10-8
E)0.54
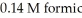 acid and
acid and 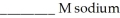 formate. The
formate. The 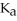 of formic acid is
of formic acid is 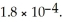
A)1.1
B)2.1
C)5.4 × 10-6
D)3.0 × 10-8
E)0.54

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck


