Deck 18: Chemistry of the Environment
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/126
Play
Full screen (f)
Deck 18: Chemistry of the Environment
1
In the troposphere,temperature ________ with increasing altitude,while in the stratosphere,temperature ________ with increasing altitude.
A)increases, increases
B)decreases, decreases
C)increases, decreases
D)decreases, increases
E)decreases, remains constant
A)increases, increases
B)decreases, decreases
C)increases, decreases
D)decreases, increases
E)decreases, remains constant
decreases, increases
2
Why are chlorofluorocarbons so damaging to the ozone layer when they are such stable molecules?
A)They contain a double bond that ozone readily attacks, resulting in the destruction of the ozone.
B)They are very light molecules that rapidly diffuse into the upper atmosphere and block the radiation that causes formation of ozone.
C)They are greenhouse gases that raise the temperature above the dissociation temperature of ozone.
D)The radiation in the stratosphere dissociates them producing chlorine atoms that catalytically destroy ozone.
E)CFCs do not damage the ozone.
A)They contain a double bond that ozone readily attacks, resulting in the destruction of the ozone.
B)They are very light molecules that rapidly diffuse into the upper atmosphere and block the radiation that causes formation of ozone.
C)They are greenhouse gases that raise the temperature above the dissociation temperature of ozone.
D)The radiation in the stratosphere dissociates them producing chlorine atoms that catalytically destroy ozone.
E)CFCs do not damage the ozone.
The radiation in the stratosphere dissociates them producing chlorine atoms that catalytically destroy ozone.
3
The majority of ozone that protects against the high energy radiation of the sun is found in the ________.
A)thermosphere
B)mesosphere
C)mesopause
D)stratosphere
E)troposphere
A)thermosphere
B)mesosphere
C)mesopause
D)stratosphere
E)troposphere
stratosphere
4
Why does the upper atmosphere contain only very little dissociated nitrogen?
A)Most of the nitrogen is in the troposphere and not in the upper atmosphere.
B)The dissociated nitrogen very rapidly diffuses out of the atmosphere and into space.
C)Nitrogen atoms are extremely reactive and so react with other substances immediately upon their formation.
D)The bond energy of nitrogen is very high and it does not absorb radiation very efficiently.
E)There is no N2 in the upper atmosphere.
A)Most of the nitrogen is in the troposphere and not in the upper atmosphere.
B)The dissociated nitrogen very rapidly diffuses out of the atmosphere and into space.
C)Nitrogen atoms are extremely reactive and so react with other substances immediately upon their formation.
D)The bond energy of nitrogen is very high and it does not absorb radiation very efficiently.
E)There is no N2 in the upper atmosphere.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is released by the combustion of fossil fuels?
A)N2
B)CO2
C)H2
D)O2
E)O3
A)N2
B)CO2
C)H2
D)O2
E)O3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
6
Of the reactions involved in the photodecomposition of ozone (shown below),which are photochemical?
1.O2 (g)+ hν → O (g)+ O (g)
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
A)2 and 4
B)1, 2, and 4
C)1 and 3
D)1 only
E)all of them
1.O2 (g)+ hν → O (g)+ O (g)
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
A)2 and 4
B)1, 2, and 4
C)1 and 3
D)1 only
E)all of them

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
7
Of the reactions involved in the photodecomposition of ozone (shown below),which are exothermic? 1.O2 (g)+ hν → O (g)+ O (g)
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
A)2 and 4
B)1 and 3
C)1, 2, and 4
D)2 only
E)all of them
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
A)2 and 4
B)1 and 3
C)1, 2, and 4
D)2 only
E)all of them

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is arranged correctly in order of increasing distance from Earth's surface?
A)mesosphere < troposphere < stratosphere < thermosphere
B)troposphere < mesosphere < stratosphere < thermosphere
C)troposphere < mesosphere < thermosphere < stratosphere
D)troposphere < stratosphere < mesosphere < thermosphere
E)mesosphere < troposphere < thermosphere < stratosphere
A)mesosphere < troposphere < stratosphere < thermosphere
B)troposphere < mesosphere < stratosphere < thermosphere
C)troposphere < mesosphere < thermosphere < stratosphere
D)troposphere < stratosphere < mesosphere < thermosphere
E)mesosphere < troposphere < thermosphere < stratosphere

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
9
Photoionization processes (e.g.,N2 + hν → N2+ + e-)remove UV of < 150 nm.Which photoreaction is the principal absorber of UV in the 150-200 nm range in the upper atmosphere?
A)N2 + hν → 2N
B)O2 + hν → 2O
C)O3 + hν → O2 + O
D)N2 + O2 + hν → 2NO
E)NO + O2 + hν → NO3
A)N2 + hν → 2N
B)O2 + hν → 2O
C)O3 + hν → O2 + O
D)N2 + O2 + hν → 2NO
E)NO + O2 + hν → NO3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
10
Cl atoms formed via photolysis of C-Cl bonds of chlorofluorocarbons in the stratosphere are particularly effective in destroying ozone at these altitudes because ________.
A)Cl atoms absorb UV, which generate O atoms to react with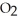 to produce ozone
to produce ozone
B)Cl atoms catalytically convert O3 to O2
C)Cl atoms stoichiometrically convert O3 to O2
D)Cl atoms react with H atoms, which catalyze conversion of O2 to O3
E)Cl atoms react with N atoms, which catalyze conversion of O2 to O3
A)Cl atoms absorb UV, which generate O atoms to react with
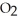 to produce ozone
to produce ozoneB)Cl atoms catalytically convert O3 to O2
C)Cl atoms stoichiometrically convert O3 to O2
D)Cl atoms react with H atoms, which catalyze conversion of O2 to O3
E)Cl atoms react with N atoms, which catalyze conversion of O2 to O3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
11
Of the compounds below,the one that requires the shortest wavelength for photoionization is ________.
A)O
B)O2
C)NO
D)N2
E)They all require the same wavelength.
A)O
B)O2
C)NO
D)N2
E)They all require the same wavelength.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
12
Why does ozone not form in high concentrations in the atmosphere above 50 km?
A)Insufficient oxygen is available.
B)Insufficient molecules exist for removal of excess energy from ozone upon its formation.
C)Light of the required wavelength is not available at those altitudes.
D)Atomic oxygen concentration is too low at high altitudes.
E)The pressure is too high.
A)Insufficient oxygen is available.
B)Insufficient molecules exist for removal of excess energy from ozone upon its formation.
C)Light of the required wavelength is not available at those altitudes.
D)Atomic oxygen concentration is too low at high altitudes.
E)The pressure is too high.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
13
The source(s)of sulfur dioxide in the atmosphere is/are ________.
A)volcanic gases
B)forest fires
C)bacterial action
D)fossil-fuel combustion
E)all of the above
A)volcanic gases
B)forest fires
C)bacterial action
D)fossil-fuel combustion
E)all of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
14
Select the substance that is thought to be partially responsible for depleting the concentration of ozone in the stratosphere.
A)CFCl3
B)CO2
C)O2
D)N2
E)He
A)CFCl3
B)CO2
C)O2
D)N2
E)He

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
15
Of the following,only ________ does not result in the formation of acid rain.
A)carbon dioxide
B)nitrogen dioxide
C)sulfur dioxide
D)nitrogen monoxide
E)methane
A)carbon dioxide
B)nitrogen dioxide
C)sulfur dioxide
D)nitrogen monoxide
E)methane

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following is a source of carbon dioxide in the troposphere?
A)natural gas seepage
B)electrical discharges
C)fossil-fuel combustion
D)volcanic gases
E)forest fires
A)natural gas seepage
B)electrical discharges
C)fossil-fuel combustion
D)volcanic gases
E)forest fires

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
17
As one gains altitude in the atmosphere,based on the ionization energies shown below,which sequence of mole fractions is the correct one?
Process Ionization Energy (kJ/mol)
N2 + hν → N2+ + e- 1495
O2 + hν → O2+ + e- 1205
O + hν → O+ + e- 1313
NO + hν → NO+ + e- 890
A)N2 > N > NO > O2
B)N2 > O > O2 > NO
C)N2 > O2 > O > NO
D)NO > O2 > O > N2
E)All will be equal.
Process Ionization Energy (kJ/mol)
N2 + hν → N2+ + e- 1495
O2 + hν → O2+ + e- 1205
O + hν → O+ + e- 1313
NO + hν → NO+ + e- 890
A)N2 > N > NO > O2
B)N2 > O > O2 > NO
C)N2 > O2 > O > NO
D)NO > O2 > O > N2
E)All will be equal.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
18
In the reactions involved in the photodecomposition of ozone (shown below),what does M symbolize?
1.O2 (g)+ hν → O (g)+ O (g)
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
A)mesosphere
B)metal
C)molybdenum
D)methane
E)molecule
1.O2 (g)+ hν → O (g)+ O (g)
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
A)mesosphere
B)metal
C)molybdenum
D)methane
E)molecule

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
19
In the past,CFCs were used in which of the following?
A)spray cans
B)plastic manufacturing
C)air conditioners
D)refrigerators
E)all of the above
A)spray cans
B)plastic manufacturing
C)air conditioners
D)refrigerators
E)all of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
20
Sulfur released into the troposphere arises from both natural and human activity.What is the ratio of human to natural sources of sulfur compounds?
A)3:1
B)2:1
C)1:1
D)1:2
E)1:3
A)3:1
B)2:1
C)1:1
D)1:2
E)1:3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
21
The lime-soda process is ________ being added to water to cause precipitation of magnesium as Mg(OH)2 and is used for large-scale water-softening operations.
A)Fe2O3
B)Al(OH)3
C)CaO
D)Fe2MgO4
E)Na2O
A)Fe2O3
B)Al(OH)3
C)CaO
D)Fe2MgO4
E)Na2O

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following substances found in the atmosphere will absorb radiation in the infrared portion of the spectrum?
A)N2
B)O2
C)Kr
D)H2O
E)He
A)N2
B)O2
C)Kr
D)H2O
E)He

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
23
The main ionic constituent of sea water is ________.
A)Na+
B)Cl-
C)SO42-
D)Mg2+
E)Ca2+
A)Na+
B)Cl-
C)SO42-
D)Mg2+
E)Ca2+

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
24
The concentration of Na in seawater is 0.470 M.How many grams of Na can be extracted from 10.0 kg of seawater if the recovery rate is 60.0%?
A)0.470
B)64.8
C)4.70
D)108
E)43.2
A)0.470
B)64.8
C)4.70
D)108
E)43.2

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
25
What is meant by the salinity of seawater?
A)percent by mass of salt in seawater
B)mass in grams of dry salts present in 1 kg of seawater
C)molality of NaCl in seawater
D)osmotic pressure of seawater
E)molarity of NaCl in seawater
A)percent by mass of salt in seawater
B)mass in grams of dry salts present in 1 kg of seawater
C)molality of NaCl in seawater
D)osmotic pressure of seawater
E)molarity of NaCl in seawater

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
26
Water containing high concentrations of ________ cations is called hard water.
A)Ca2+
B)Mg2+
C)Na+
D)K+
E)Ca2+ or Mg2+
A)Ca2+
B)Mg2+
C)Na+
D)K+
E)Ca2+ or Mg2+

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
27
Which gaseous sulfur compound combines with water to form the principal acidic constituent of acid rain?
A)SO42-
B)SO3
C)SO2
D)SO
E)H2S
A)SO42-
B)SO3
C)SO2
D)SO
E)H2S

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
28
Chemical treatment of municipal water supplies commonly entails use of CaO,Al2(SO4)3,and Cl2.The purpose of adding CaO is to ________.
A)remove all HCO3- as solid CaCO3
B)remove all SO42- as solid CaSO4
C)remove all Cl- as solid CaCl2
D)selectively kill anaerobic (but not aerobic)bacteria
E)make the water slightly basic so that addition of Al2(SO4)3 will afford a gelatinous precipitate of Al(OH)3
A)remove all HCO3- as solid CaCO3
B)remove all SO42- as solid CaSO4
C)remove all Cl- as solid CaCl2
D)selectively kill anaerobic (but not aerobic)bacteria
E)make the water slightly basic so that addition of Al2(SO4)3 will afford a gelatinous precipitate of Al(OH)3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
29
A single individual typically uses the greatest quantity of water for ________.
A)flushing toilets
B)cooking
C)cleaning (bathing, laundering, and house cleaning)
D)watering lawns
E)drinking water
A)flushing toilets
B)cooking
C)cleaning (bathing, laundering, and house cleaning)
D)watering lawns
E)drinking water

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
30
Incomplete combustion of carbon-containing materials occurs when ________.
A)there is insufficient oxygen to convert all of the carbon to carbon dioxide
B)there are sulfur impurities in the carbon-containing material
C)the carbon-containing material is a gas
D)the combustion flame is too hot
E)there is an excess of oxygen
A)there is insufficient oxygen to convert all of the carbon to carbon dioxide
B)there are sulfur impurities in the carbon-containing material
C)the carbon-containing material is a gas
D)the combustion flame is too hot
E)there is an excess of oxygen

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
31
THMs are ________.
A)non-toxic
B)natural
C)used in green chemistry
D)suspected carcinogens
E)atmospheric pollutants
A)non-toxic
B)natural
C)used in green chemistry
D)suspected carcinogens
E)atmospheric pollutants

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is classified as a supercritical fluid?
A)water
B)xylene
C)toluene
D)hydrogen peroxide
E)none of the above
A)water
B)xylene
C)toluene
D)hydrogen peroxide
E)none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
33
Carbon dioxide contributes to atmospheric warming by ________.
A)absorbing incoming radiation from the sun and converting it to heat
B)absorbing radiation emitted from the surface of the earth preventing its loss to space
C)undergoing exothermic reactions extensively in the atmosphere
D)increasing the index of refraction of the atmosphere so that infrared radiation from the sun is refracted to the surface of the earth where it is converted to heat
E)reducing the concentration of CO in the atmosphere
A)absorbing incoming radiation from the sun and converting it to heat
B)absorbing radiation emitted from the surface of the earth preventing its loss to space
C)undergoing exothermic reactions extensively in the atmosphere
D)increasing the index of refraction of the atmosphere so that infrared radiation from the sun is refracted to the surface of the earth where it is converted to heat
E)reducing the concentration of CO in the atmosphere

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
34
To produce acceptable quality drinking water from seawater by desalination,the level of salt must be reduced ________ fold.
A)10
B)50
C)70
D)100
E)120
A)10
B)50
C)70
D)100
E)120

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
35
How does lime reduce sulfur dioxide emissions from the burning of coal?
A)It reacts with the sulfur dioxide to form calcium sulfite solid that can be precipitated.
B)It reduces the sulfur dioxide to elemental sulfur that is harmless to the environment.
C)It oxidizes the sulfur dioxide to tetrathionate that is highly water soluble so it can be scrubbed from the emission gases.
D)It catalyzes the conversion of sulfur dioxide to sulfur trioxide which is much less volatile and can be removed by condensation.
E)It converts S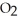 to solid, elemental sulfur.
to solid, elemental sulfur.
A)It reacts with the sulfur dioxide to form calcium sulfite solid that can be precipitated.
B)It reduces the sulfur dioxide to elemental sulfur that is harmless to the environment.
C)It oxidizes the sulfur dioxide to tetrathionate that is highly water soluble so it can be scrubbed from the emission gases.
D)It catalyzes the conversion of sulfur dioxide to sulfur trioxide which is much less volatile and can be removed by condensation.
E)It converts S
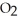 to solid, elemental sulfur.
to solid, elemental sulfur.
Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is not a stage in water treatment?
A)coarse filtration
B)aeration
C)chlorination
D)distillation
E)settling
A)coarse filtration
B)aeration
C)chlorination
D)distillation
E)settling

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
37
The cumulative result of carbon dioxide,methane,and ozone in the troposphere on atmospheric temperatures is referred to as ________.
A)acid rain
B)the greenhouse effect
C)photoionization
D)photochemical smog
E)photodissociation
A)acid rain
B)the greenhouse effect
C)photoionization
D)photochemical smog
E)photodissociation

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
38
Eutrophication of a lake is the process of ________.
A)rapid increase in the amount of dead and decaying plant matter in the lake as a result of excessive plant growth
B)rapid decline in the lake's pH due to acid rain
C)dissolved oxygen being depleted by an overpopulation of fish
D)stocking the lake with fish
E)restoration of the lake's dissolved oxygen supply by aerobic bacteria
A)rapid increase in the amount of dead and decaying plant matter in the lake as a result of excessive plant growth
B)rapid decline in the lake's pH due to acid rain
C)dissolved oxygen being depleted by an overpopulation of fish
D)stocking the lake with fish
E)restoration of the lake's dissolved oxygen supply by aerobic bacteria

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction that forms most of the acid in acid rain is ________.
A)SO2 (g)+ H2O (l)→ H2SO4 (aq)
B)SO2 (g)+ H2O (l)→ H2SO3 (aq)
C)H2S (g)+ 2 O2 (g)→ H2SO4 (l)
D)Cl2 (g)+ H2O (l)→ HCl (aq)+ HClO (aq)
E)SO3 (g)+ H2O (l)→ H2SO4 (aq)
A)SO2 (g)+ H2O (l)→ H2SO4 (aq)
B)SO2 (g)+ H2O (l)→ H2SO3 (aq)
C)H2S (g)+ 2 O2 (g)→ H2SO4 (l)
D)Cl2 (g)+ H2O (l)→ HCl (aq)+ HClO (aq)
E)SO3 (g)+ H2O (l)→ H2SO4 (aq)

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
40
The primary detrimental effect of the presence of large amounts of biodegradable organic materials in water is ________.
A)it causes death of bottom dwelling organisms because it agglutinates and settles to the bottom, poisoning bottom dwelling organisms
B)it causes oxygen depletion in the water
C)it rises to the surface and absorbs wavelengths needed by aquatic plants
D)it decomposes endothermically causing the temperature of the water to decrease below the limits within which most aquatic organisms can live
E)it causes the water to become murky
A)it causes death of bottom dwelling organisms because it agglutinates and settles to the bottom, poisoning bottom dwelling organisms
B)it causes oxygen depletion in the water
C)it rises to the surface and absorbs wavelengths needed by aquatic plants
D)it decomposes endothermically causing the temperature of the water to decrease below the limits within which most aquatic organisms can live
E)it causes the water to become murky

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
41
CFC stands for ________.
A)chlorinated freon compound
B)chlorofluorocarbon
C)carbonated fluorine compound
D)caustic fluorine carbohydrate
E)carbofluoro compound
A)chlorinated freon compound
B)chlorofluorocarbon
C)carbonated fluorine compound
D)caustic fluorine carbohydrate
E)carbofluoro compound

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
42
Radiation in the infrared portion of the spectrum is absorbed by ________ found in the atmosphere.
A)N2
B)O2
C)CO2
D)Ar
E)He
A)N2
B)O2
C)CO2
D)Ar
E)He

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
43
Ozone is a(n)________ of oxygen.
A)isomer
B)allotrope
C)isotope
D)resonance structure
E)atomic form
A)isomer
B)allotrope
C)isotope
D)resonance structure
E)atomic form

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
44
The pressure of the atmosphere ________.
A)increases with altitude
B)follows the same trend as temperature
C)decreases with altitude
D)follows the reverse trend as temperature
E)stays the same
A)increases with altitude
B)follows the same trend as temperature
C)decreases with altitude
D)follows the reverse trend as temperature
E)stays the same

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
45
Ozone is a necessary,protective component of the ________,but is considered a pollutant in the ________.
A)troposphere, upper atmosphere
B)troposphere, air
C)photochemical smog, air we breathe
D)upper atmosphere, troposphere
E)air we breathe, upper atmosphere
A)troposphere, upper atmosphere
B)troposphere, air
C)photochemical smog, air we breathe
D)upper atmosphere, troposphere
E)air we breathe, upper atmosphere

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
46
The dividing line between the troposphere and stratosphere is known as the ________.
A)mesosphere
B)tropopause
C)thermosphere
D)stratopause
E)mesopause
A)mesosphere
B)tropopause
C)thermosphere
D)stratopause
E)mesopause

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
47
What is/are the product(s)of photodissociation of molecular oxygen?
A)molecular nitrogen
B)excited oxygen molecules
C)ozone
D)ozone and atomic oxygen
E)atomic oxygen
A)molecular nitrogen
B)excited oxygen molecules
C)ozone
D)ozone and atomic oxygen
E)atomic oxygen

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
48
The majority of SO2 released annually in the United States results from ________.
A)combustion of oil
B)acid rain
C)combustion of coal
D)depletion of ozone by CFCs
E)all of the above
A)combustion of oil
B)acid rain
C)combustion of coal
D)depletion of ozone by CFCs
E)all of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
49
Natural,unpolluted rainwater is typically acidic.What is the source of this natural acidity?
A)CO2
B)SO2
C)NO2
D)HCl
E)chlorofluorocarbons
A)CO2
B)SO2
C)NO2
D)HCl
E)chlorofluorocarbons

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
50
The C-Cl and C-F bond dissociation energies in CF3Cl are 339 kJ/mol and 482 kJ/mol,respectively.The maximum wavelengths of electromagnetic radiation required to rupture these bonds are ________ and ________,respectively.
A)45.0 nm, 307 nm
B)742 nm, 654 nm
C)482 nm, 248 nm
D)353 nm, 248 nm
E)979 nm, 953 nm
A)45.0 nm, 307 nm
B)742 nm, 654 nm
C)482 nm, 248 nm
D)353 nm, 248 nm
E)979 nm, 953 nm

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
51
What compound in limestone and marble is attacked by acid rain?
A)hydroxyapatite
B)calcium carbonate
C)gypsum
D)graphite
E)potassium hydroxide
A)hydroxyapatite
B)calcium carbonate
C)gypsum
D)graphite
E)potassium hydroxide

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
52
The amount of atomic O relative to O2 ________.
A)is highest in the troposphere
B)is highest in the stratosphere
C)increases with altitude in the thermosphere
D)decreases with altitude in the thermosphere
E)is essentially independent of altitude in the thermosphere
A)is highest in the troposphere
B)is highest in the stratosphere
C)increases with altitude in the thermosphere
D)decreases with altitude in the thermosphere
E)is essentially independent of altitude in the thermosphere

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
53
Compounds found in fossil fuels that contain ________ are primarily responsible for acid rain.
A)sulfur
B)carbon
C)hydrogen
D)phosphorus
E)neon
A)sulfur
B)carbon
C)hydrogen
D)phosphorus
E)neon

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
54
The layer of the atmosphere that contains our weather is called the ________.
A)mesosphere
B)heterosphere
C)stratosphere
D)thermosphere
E)troposphere
A)mesosphere
B)heterosphere
C)stratosphere
D)thermosphere
E)troposphere

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
55
Of the noble gases,________ is present in highest concentration in dry air at sea level.
A)Ne
B)He
C)Xe
D)Kr
E)Ar
A)Ne
B)He
C)Xe
D)Kr
E)Ar

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
56
CO2 from hydrocarbon combustion creates a major environmental problem that is described as ________.
A)the greenhouse effect
B)photochemical smog
C)acid rain
D)stratospheric ozone depletion
E)all of the above
A)the greenhouse effect
B)photochemical smog
C)acid rain
D)stratospheric ozone depletion
E)all of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
57
The C  O bond dissociation energy in CO2 is 799 kJ/mol.The maximum wavelength of electromagnetic radiation required to rupture this bond is ________.
O bond dissociation energy in CO2 is 799 kJ/mol.The maximum wavelength of electromagnetic radiation required to rupture this bond is ________.
A)307 nm
B)149 nm
C)248 nm
D)353 nm
E)979 nm
 O bond dissociation energy in CO2 is 799 kJ/mol.The maximum wavelength of electromagnetic radiation required to rupture this bond is ________.
O bond dissociation energy in CO2 is 799 kJ/mol.The maximum wavelength of electromagnetic radiation required to rupture this bond is ________.A)307 nm
B)149 nm
C)248 nm
D)353 nm
E)979 nm

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
58
In the equation below,M is most likely ________. O + O2 + M → O3 + M*
A)Br2
B)Xe
C)SO2
D)N2
E)H2O
A)Br2
B)Xe
C)SO2
D)N2
E)H2O

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
59
Acid rain typically has a pH of about ________.
A)7
B)5
C)4
D)2
E)1
A)7
B)5
C)4
D)2
E)1

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
60
Sulfur dioxide is not released into the atmosphere in any significant amount by ________.
A)burning of coal
B)bacterial action
C)internal combustion engines
D)volcanic eruption
E)Sulfur dioxide is produced in significant amount by all of these processes.
A)burning of coal
B)bacterial action
C)internal combustion engines
D)volcanic eruption
E)Sulfur dioxide is produced in significant amount by all of these processes.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
61
The concentration of which greenhouse gas has increased steadily over the last few decades?
A)H2O
B)CO
C)CO2
D)H2O2
E)O2
A)H2O
B)CO
C)CO2
D)H2O2
E)O2

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
62
What is the total pressure (torr)inside a container that contains a gas at 8.57 × 103 ppm where its partial pressure is 1.87 torr?
A)4.58 × 109
B)1.60 × 10-2
C)2.18 × 10-4
D)218
E)0.218
A)4.58 × 109
B)1.60 × 10-2
C)2.18 × 10-4
D)218
E)0.218

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
63
The "scale" caused by hard water is ________.
A)calcium ions
B)sodium chloride
C)magnesium oxide
D)sodium ions
E)calcium carbonate
A)calcium ions
B)sodium chloride
C)magnesium oxide
D)sodium ions
E)calcium carbonate

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
64
What is the total pressure (torr)inside a container that contains a gas at 3.18 × 103 ppm where its partial pressure is 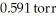 ?
?
A)5.38 × 10-3
B)1.86 × 10-4
C)1.88 × 103
D)1.86 × 10-10
E)1.88 × 109
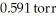 ?
?A)5.38 × 10-3
B)1.86 × 10-4
C)1.88 × 103
D)1.86 × 10-10
E)1.88 × 109

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
65
The concentration of ozone in a sample of air that has a partial pressure of O3 of 0.53 torr and a total pressure of air of 695 torr is ________ ppm.
A)7.6 ×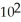
B)0.76
C)0.076
D)1.3 ×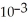
E)1.3
A)7.6 ×
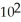
B)0.76
C)0.076
D)1.3 ×
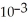
E)1.3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
66
What is the concentration (ppm)of helium in the atmosphere if the mole fraction of helium in dry air near sea level is 0.00000524?
A)5.24
B)1.91 × 1011
C)5.24 × 10-4
D)5240
E)5.24 × 10-12
A)5.24
B)1.91 × 1011
C)5.24 × 10-4
D)5240
E)5.24 × 10-12

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
67
What is the concentration (ppm)of water vapor in a sample of air that has a partial pressure of water of 1.20 torr and a total pressure of air of 695 torr?
A)1.7
B)0.17
C)5.8 ×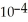
D)1.7 ×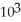
E)0.58
A)1.7
B)0.17
C)5.8 ×
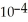
D)1.7 ×
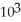
E)0.58

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is (are)not a typical source of nitric oxide?
A)livestock emissions
B)atmospheric electrical discharges
C)internal combustion engines
D)combustion of organic matter
E)all of the above
A)livestock emissions
B)atmospheric electrical discharges
C)internal combustion engines
D)combustion of organic matter
E)all of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
69
The brown color of photochemical smog over a city is mainly due to ________.
A)NO2
B)N2O4
C)CO
D)SO2
E)CO2
A)NO2
B)N2O4
C)CO
D)SO2
E)CO2

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
70
The mole fraction of nitrogen in dry air near sea level is 0.78084,where the molar mass of nitrogen is 28.013.The partial pressure of nitrogen when the total atmospheric pressure (dry air)is 97.5 kPa is 
A)126
B)4.51
C)77.0
D)2.16 × 103
E)2.75

A)126
B)4.51
C)77.0
D)2.16 × 103
E)2.75

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
71
Components of Air Mole Fraction Nitrogen 0.781
Oxygen 0.209
Argon 0.010
What is the partial pressure or nitrogen (in torr)in the atmosphere when the atmospheric pressure is 760.0 torr?
A)159
B)594
C)611
D)7.60
E)760
Oxygen 0.209
Argon 0.010
What is the partial pressure or nitrogen (in torr)in the atmosphere when the atmospheric pressure is 760.0 torr?
A)159
B)594
C)611
D)7.60
E)760

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
72
Components of Air Mole Fraction Nitrogen 0.781
Oxygen 0.209
Argon 0.010
What is the partial pressure or oxygen (in torr)in the atmosphere when the atmospheric pressure is 755.0 torr?
A)158
B)590.
C)611
D)7.55
E)760
Oxygen 0.209
Argon 0.010
What is the partial pressure or oxygen (in torr)in the atmosphere when the atmospheric pressure is 755.0 torr?
A)158
B)590.
C)611
D)7.55
E)760

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
73
What is the wavelength of a photon with just enough energy to break a 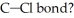 (The bond energy of the
(The bond energy of the 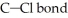 is
is 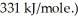
A)362 nm
B)723 nm
C)181 nm
D)5.46 × 1035 nm
E)5.50 × 10-19 nm
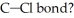 (The bond energy of the
(The bond energy of the 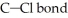 is
is 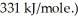
A)362 nm
B)723 nm
C)181 nm
D)5.46 × 1035 nm
E)5.50 × 10-19 nm

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
74
The concentration of CO in a room is 7.2 ppm.If the total pressure is 695 torr,then the partial pressure of CO is ________ torr.
A)5.0 ×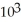
B)5.0
C)1.0 × 104
D)5.0 ×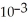
E)9.7 × 107
A)5.0 ×
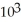
B)5.0
C)1.0 × 104
D)5.0 ×
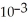
E)9.7 × 107

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
75
The mole fraction of nitrous oxide in dry air near sea level is 0.00000050.The concentration of nitrous oxide is ________ molecules per liter,assuming an atmospheric pressure of 739 torr and a temperature of 29.5 °C.
A)1.5 × 10-5
B)2.0 × 10-8
C)1.2 × 1016
D)8.9 × 1018
E)1.2 × 1017
A)1.5 × 10-5
B)2.0 × 10-8
C)1.2 × 1016
D)8.9 × 1018
E)1.2 × 1017

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
76
In the world's oceans,the average salinity is about ________ g/kg.
A)0.03
B)0.1
C)35
D)17
E)3.5
A)0.03
B)0.1
C)35
D)17
E)3.5

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
77
The concentration of carbon monoxide in a sample of air is 6.0 ppm.There are ________ molecules of CO in 1.00 L of this air at 755 torr and 23 °C.
A)2.5 ×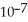
B)1.4 ×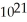
C)1.9 ×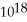
D)1.1 ×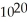
E)1.5 ×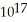
A)2.5 ×
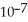
B)1.4 ×
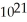
C)1.9 ×
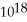
D)1.1 ×
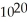
E)1.5 ×
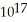

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
78
The area of the Earth's atmosphere 50 - 85 km above the surface is called the ________.
A)mesosphere
B)thermosphere
C)troposphere
D)stratosphere
E)exosphere
A)mesosphere
B)thermosphere
C)troposphere
D)stratosphere
E)exosphere

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
79
What is the percentage of freshwater on planet Earth?
A)97%
B)2.1%
C)2.8%
D)0.6%
E)100%
A)97%
B)2.1%
C)2.8%
D)0.6%
E)100%

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
80
The sterilizing action of chlorine in water is due to what substance?
A)Cl-
B)Cl2
C)HCl
D)HClO
E)H+
A)Cl-
B)Cl2
C)HCl
D)HClO
E)H+

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck


