Deck 3: Chemical Reactions and Reaction Stoichiometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/190
Play
Full screen (f)
Deck 3: Chemical Reactions and Reaction Stoichiometry
1
Which of the following are decomposition reactions?
1. CH4 (g) + O2 (g) CO2 (g) + H2O (l)
2. CaO (s) + CO2 (g) CaCO3 (s)
3. Mg (s) + O2(g) MgO (s)
4. PbCO3 (s) PbO (s) + CO2 (g)
A) 1, 2, and 3
B) 4 only
C) 1, 2, 3, and 4
D) 2 and 3
E) 2, 3, and 4
1. CH4 (g) + O2 (g) CO2 (g) + H2O (l)
2. CaO (s) + CO2 (g) CaCO3 (s)
3. Mg (s) + O2(g) MgO (s)
4. PbCO3 (s) PbO (s) + CO2 (g)
A) 1, 2, and 3
B) 4 only
C) 1, 2, 3, and 4
D) 2 and 3
E) 2, 3, and 4
4 only
2
When a hydrocarbon burns in air,what component of air reacts?
A)oxygen
B)nitrogen
C)carbon dioxide
D)water
E)argon
A)oxygen
B)nitrogen
C)carbon dioxide
D)water
E)argon
oxygen
3
The molecular weight of the acetic acid (CH3CO2H),rounded to the nearest integer,is ________ amu.
A)60
B)48
C)44
D)32
A)60
B)48
C)44
D)32
60
4
When a hydrocarbon burns in air,a component produced is ________.
A)oxygen
B)nitrogen
C)carbon
D)water
E)argon
A)oxygen
B)nitrogen
C)carbon
D)water
E)argon

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
5
The formula weight of potassium dichromate (K2Cr2O7)is ________ amu.
A)107.09
B)255.08
C)242.18
D)294.18
E)333.08
A)107.09
B)255.08
C)242.18
D)294.18
E)333.08

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
6
Of the reactions below,which one is not a combination reaction?
A)C + O2 → CO2
B)2Mg + O2 → 2MgO
C)2N2 + 3H2 → 2NH3
D)CaO + H2O → Ca(OH)2
E)2CH4 + 4O2 → 2CO2 + 4H2O
A)C + O2 → CO2
B)2Mg + O2 → 2MgO
C)2N2 + 3H2 → 2NH3
D)CaO + H2O → Ca(OH)2
E)2CH4 + 4O2 → 2CO2 + 4H2O

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following are combustion reactions?
1. CH4 (g) + O2 (g) → CO2 (g) + H2O (l)
2. CaO (s) + CO2 (g) → CaCO3 (s)
3. PbCO3 (s) → PbO (s) + CO2 (g)
4. CH3OH (l) + O2 (g) → CO2 (g) + H2O (l)
A) 1 and 4
B) 1, 2, 3, and 4
C) 1, 3, and 4
D) 2, 3, and 4
E) 3 and 4
1. CH4 (g) + O2 (g) → CO2 (g) + H2O (l)
2. CaO (s) + CO2 (g) → CaCO3 (s)
3. PbCO3 (s) → PbO (s) + CO2 (g)
4. CH3OH (l) + O2 (g) → CO2 (g) + H2O (l)
A) 1 and 4
B) 1, 2, 3, and 4
C) 1, 3, and 4
D) 2, 3, and 4
E) 3 and 4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
8
The formula weight of silver chromate (Ag2CrO4)is ________ amu.
A)159.87
B)223.87
C)331.73
D)339.86
E)175.87
A)159.87
B)223.87
C)331.73
D)339.86
E)175.87

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
9
What is the formula weight of lead iodide (PbI2)?
A)334.1
B)168.7
C)295.6
D)461.0
E)668.2
A)334.1
B)168.7
C)295.6
D)461.0
E)668.2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
10
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
10
What is the formula weight of magnesium fluoride (MgF2)?
A)86.6
B)43.3
C)62.3
D)67.6
E)92.9
A)86.6
B)43.3
C)62.3
D)67.6
E)92.9

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
11
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
11
The formula of nitrobenzene is C6H5NO2.The molecular weight of this compound is ________ amu.
A)107.11
B)43.03
C)109.10
D)123.11
E)3.06
A)107.11
B)43.03
C)109.10
D)123.11
E)3.06

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
12
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
12
When the following equation is balanced,the coefficients are ________. C8H18 + O2 → CO2 + H2O
A)2, 3, 4, 4
B)1, 4, 8, 9
C)2, 12, 8, 9
D)4, 4, 32, 36
E)2, 25, 16, 18
A)2, 3, 4, 4
B)1, 4, 8, 9
C)2, 12, 8, 9
D)4, 4, 32, 36
E)2, 25, 16, 18

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
13
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
13
The reaction used to inflate automobile airbags ________.
A)produces sodium gas
B)is a combustion reaction
C)is a combination reaction
D)violates the law of conservation of mass
E)is a decomposition reaction
A)produces sodium gas
B)is a combustion reaction
C)is a combination reaction
D)violates the law of conservation of mass
E)is a decomposition reaction

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
14
The formula weight of ammonium sulfate ((NH4)2SO4),rounded to the nearest integer,is ________ amu.
A)100
B)118
C)116
D)132
E)264
A)100
B)118
C)116
D)132
E)264

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
15
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following are combination reactions?
1.CH4 (g) + O2 (g) → CO2 (g) + H2O (l)
2.CaO (s) + CO2 (g) → CaCO3 (s)
3.Mg (s) + O2(g) → MgO (s)
4.PbCO3 (s) → PbO (s) + CO2 (g)
A) 1, 2, and 3
B) 2 and 3
C) 1, 2, 3, and 4
D) 4 only
E) 2, 3, and 4
1.CH4 (g) + O2 (g) → CO2 (g) + H2O (l)
2.CaO (s) + CO2 (g) → CaCO3 (s)
3.Mg (s) + O2(g) → MgO (s)
4.PbCO3 (s) → PbO (s) + CO2 (g)
A) 1, 2, and 3
B) 2 and 3
C) 1, 2, 3, and 4
D) 4 only
E) 2, 3, and 4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
16
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
16
Of the reactions below,which one is a decomposition reaction?
A)NH4Cl → NH3 + HCl
B)2Mg + O2 → 2MgO
C)2N2 + 3H2 → 2NH3
D)2CH4 + 4O2 → 2CO2 + 4H2O
E)Cd(NO3)2 + Na2S → CdS + 2NaNO3
A)NH4Cl → NH3 + HCl
B)2Mg + O2 → 2MgO
C)2N2 + 3H2 → 2NH3
D)2CH4 + 4O2 → 2CO2 + 4H2O
E)Cd(NO3)2 + Na2S → CdS + 2NaNO3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
17
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following substances is the product of this combination reaction?
Al (s)+ I2(s)→ ________
A)AlI2
B)AlI
C)AlI3
D)Al2I3
E)Al3I2
Al (s)+ I2(s)→ ________
A)AlI2
B)AlI
C)AlI3
D)Al2I3
E)Al3I2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
18
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
18
The formula weight of a substance is ________.
A)identical to the molar mass
B)the same as the percent by mass weight
C)determined by combustion analysis
D)the sum of the atomic weights of each atom in its chemical formula
E)the weight of a sample of the substance
A)identical to the molar mass
B)the same as the percent by mass weight
C)determined by combustion analysis
D)the sum of the atomic weights of each atom in its chemical formula
E)the weight of a sample of the substance

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
19
The formula weight of calcium nitrate (Ca(NO3)2),rounded to one decimal place,is ________ amu.
A)102.1
B)164.0
C)204.2
D)150.1
E)116.1
A)102.1
B)164.0
C)204.2
D)150.1
E)116.1

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
20
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is not true concerning automotive air bags?
A)They are inflated as a result of a decomposition reaction.
B)They are loaded with sodium azide initially.
C)The gas used for inflating them is oxygen.
D)The two products of the decomposition reaction are sodium and nitrogen.
E)A gas is produced when the air bag activates.
A)They are inflated as a result of a decomposition reaction.
B)They are loaded with sodium azide initially.
C)The gas used for inflating them is oxygen.
D)The two products of the decomposition reaction are sodium and nitrogen.
E)A gas is produced when the air bag activates.

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
21
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
21
A sample of CH4O with a mass of 32.0 g contains ________ molecules of CH4O.
A)5.32 × 10-23
B)1.00
C)1.88 × 1022
D)6.02 × 1023
E)32.0
A)5.32 × 10-23
B)1.00
C)1.88 × 1022
D)6.02 × 1023
E)32.0

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
22
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
22
The mass % of H in methane (CH4)is ________.
A)25.13
B)4.032
C)74.87
D)92.26
E)7.743
A)25.13
B)4.032
C)74.87
D)92.26
E)7.743

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
23
AA

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
23
The mass % of C in methane (CH4)is ________.
A)25.13
B)133.6
C)74.87
D)92.26
E)7.743
A)25.13
B)133.6
C)74.87
D)92.26
E)7.743

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the percentage by mass of oxygen in Pb(NO3)2.
A)9.7
B)14.5
C)19.3
D)29.0
E)33.4
A)9.7
B)14.5
C)19.3
D)29.0
E)33.4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
25
Calculate the percentage by mass of nitrogen in PtCl2(NH3)2.
A)4.67
B)9.34
C)9.90
D)4.95
E)12.67
A)4.67
B)9.34
C)9.90
D)4.95
E)12.67

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
26
The mass % of F in the binary compound KrF2 is ________.
A)18.48
B)45.38
C)68.80
D)81.52
E)31.20
A)18.48
B)45.38
C)68.80
D)81.52
E)31.20

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the percentage by mass of ammonia in cisplatin,PtCl2(NH3)2.
A)5.68
B)11.35
C)4.67
D)12.53
E)18.09
A)5.68
B)11.35
C)4.67
D)12.53
E)18.09

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
28
How many atoms of nitrogen are in 10 g of NH4NO3?
A)3.5
B)1.5 × 1023
C)3.0 × 1023
D)1.8
E)2
A)3.5
B)1.5 × 1023
C)3.0 × 1023
D)1.8
E)2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
29
How many oxygen atoms are contained in 2.74 g of Al2(SO4)3?
A)12
B)6.02 × 1023
C)7.22 × 1024
D)5.79 × 1022
E)8.01 × 10-3
A)12
B)6.02 × 1023
C)7.22 × 1024
D)5.79 × 1022
E)8.01 × 10-3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
30
What is the total number of atoms in 0.139 mol of Fe(OH2)63+?
A)19.0
B)1.59 × 1024
C)8.37 × 1022
D)2.64
E)1.84 × 1024
A)19.0
B)1.59 × 1024
C)8.37 × 1022
D)2.64
E)1.84 × 1024

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
31
One million argon atoms is ________ mol (rounded to two significant figures)of argon atoms.
A)3.0
B)1.7 × 10-18
C)6.0 × 1023
D)1.0 × 10-6
E)1.0 × 10+6
A)3.0
B)1.7 × 10-18
C)6.0 × 1023
D)1.0 × 10-6
E)1.0 × 10+6

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the percentage by mass of carbon in CO2.
A)27.29
B)75.10
C)73.05
D)72.71
E)37.53
A)27.29
B)75.10
C)73.05
D)72.71
E)37.53

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
33
One mole of ________ contains the smallest number of atoms.
A)S8
B)C10H8
C)Al2(SO4)3
D)Na3PO4
E)NaCl
A)S8
B)C10H8
C)Al2(SO4)3
D)Na3PO4
E)NaCl

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
34
How many molecules of CH4 are in 48.2 g of this compound?
A)5.00 × 10-24
B)2.00 × 1023
C)4.64 × 1026
D)1.81 × 1024
E)4.00
A)5.00 × 10-24
B)2.00 × 1023
C)4.64 × 1026
D)1.81 × 1024
E)4.00

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
35
Gaseous neon has a density of 0.900 g/L at standard conditions.How many neon atoms are in 1.00 L of neon gas at standard conditions?
A)5.42 × 1023
B)1.35 × 1025
C)7.41 × 10-26
D)2.69 × 1022
E)6.02 × 1023
A)5.42 × 1023
B)1.35 × 1025
C)7.41 × 10-26
D)2.69 × 1022
E)6.02 × 1023

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
36
A 30.5 gram sample of glucose (C6H12O6)contains ________ mol of glucose.
A)0.424
B)0.169
C)5.90
D)2.36
E)0.136
A)0.424
B)0.169
C)5.90
D)2.36
E)0.136

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following contains the largest number of atoms in mole?
A)S8
B)C4H10
C)Al2(SO4)3
D)Na3PO4
E)Cl2
A)S8
B)C4H10
C)Al2(SO4)3
D)Na3PO4
E)Cl2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of CH2F2 with a mass of 19 g contains ________ atoms of F.
A)2.2 × 1023
B)38
C)3.3 × 1024
D)4.4 × 1023
E)9.5
A)2.2 × 1023
B)38
C)3.3 × 1024
D)4.4 × 1023
E)9.5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the percentage by mass of lead in Pb(NO3)2.
A)38.6
B)44.5
C)62.6
D)65.3
E)71.2
A)38.6
B)44.5
C)62.6
D)65.3
E)71.2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the percentage by mass of hydrogen in PtCl2(NH3)2.
A)1.558
B)1.008
C)0.672
D)0.034
E)2.016
A)1.558
B)1.008
C)0.672
D)0.034
E)2.016

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
41
When the following equation is balanced,the coefficient of H2 is ________. K (s)+ H2O (l)→ KOH (aq)+ H2 (g)
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
42
Propane (C3H8)reacts with oxygen in the air to produce carbon dioxide and water.In a particular experiment,38.0 grams of carbon dioxide are produced from the reaction of 22.05 grams of propane with excess oxygen.What is the % yield in this reaction?
A)38.0
B)57.6
C)66.0
D)86.4
E)94.5
A)38.0
B)57.6
C)66.0
D)86.4
E)94.5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
43
How many sulfur dioxide molecules are there in 1.80 mol of sulfur dioxide?
A)1.08 × 1023
B)6.02 × 1024
C)1.80 × 1024
D)1.08 × 1024
E)6.02 × 1023
A)1.08 × 1023
B)6.02 × 1024
C)1.80 × 1024
D)1.08 × 1024
E)6.02 × 1023

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
44
The molecular formula of aspartame,the generic name of NutraSweet®,is C14H18N2O5.What is the mass (g)of 1.00 mol of aspartame?
A)24
B)156
C)294
D)43
E)39
A)24
B)156
C)294
D)43
E)39

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
45
When the following equation is balanced,the coefficient of H2S is ________. FeCl3 (aq)+ H2S (g)→ Fe2S3 (s)+ HCl (aq)
A)1
B)2
C)3
D)5
E)4
A)1
B)2
C)3
D)5
E)4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
46
How many carbon atoms are there in 52.06 g of carbon dioxide?
A)5.206 × 1024
B)3.134 × 1025
C)7.122 × 1023
D)8.648 × 10-23
E)1.424 × 1024
A)5.206 × 1024
B)3.134 × 1025
C)7.122 × 1023
D)8.648 × 10-23
E)1.424 × 1024

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
47
When the following equation is balanced,the coefficient of H2O is ________. Ca (s)+ H2O (l)→ Ca(OH)2 (aq)+ H2 (g)
A)1
B)2
C)3
D)5
E)4
A)1
B)2
C)3
D)5
E)4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
48
When the following equation is balanced,the coefficients are ________. NH3 (g)+ O2 (g)→ NO2 (g)+ H2O (g)
A)1, 1, 1, 1
B)4, 7, 4, 6
C)2, 3, 2, 3
D)1, 3, 1, 2
E)4, 3, 4, 3
A)1, 1, 1, 1
B)4, 7, 4, 6
C)2, 3, 2, 3
D)1, 3, 1, 2
E)4, 3, 4, 3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
49
How many oxygen atoms are there in 52.06 g of carbon dioxide?
A)1.424 × 1024
B)6.022 × 1023
C)7.122 × 1023
D)5.088 × 1023
E)1.018 × 1024
A)1.424 × 1024
B)6.022 × 1023
C)7.122 × 1023
D)5.088 × 1023
E)1.018 × 1024

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
50
Which hydrocarbon pair below has identical mass percentage of C?
A)C3H4 and C3H6
B)C2H4 and C3H4
C)C2H4 and C4H2
D)C2H4 and C3H6
E)none of the above
A)C3H4 and C3H6
B)C2H4 and C3H4
C)C2H4 and C4H2
D)C2H4 and C3H6
E)none of the above

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
51
How many grams of sodium carbonate contain 1.773 × 1017 carbon atoms?
A)3.121 × 10-5
B)1.011 × 10-5
C)1.517 × 10-5
D)9.100 × 10-5
E)6.066 × 10-5
A)3.121 × 10-5
B)1.011 × 10-5
C)1.517 × 10-5
D)9.100 × 10-5
E)6.066 × 10-5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
52
There are ________ oxygen atoms in 30 molecules of C20H42S3O2.
A)6.0 × 1023
B)1.8 × 1025
C)3.6 × 1025
D)1.2 × 1024
E)60
A)6.0 × 1023
B)1.8 × 1025
C)3.6 × 1025
D)1.2 × 1024
E)60

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
53
The compound responsible for the characteristic smell of garlic is allicin,C6H10OS2.What is the mass (g)of 1.00 mol of allicin?
A)34
B)162
C)86
D)61
E)19
A)34
B)162
C)86
D)61
E)19

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
54
When the following equation is balanced,the coefficient of Al2O3 is ________. Al2O3 (s)+ C (s)+ Cl2 (g)→ AlCl3 (s)+ CO (g)
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
55
A nitrogen oxide is 63.65% by mass nitrogen.The molecular formula could be ________.
A)NO
B)NO2
C)N2O
D)N2O4
E)either NO2 or N2O4
A)NO
B)NO2
C)N2O
D)N2O4
E)either NO2 or N2O4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
56
A sulfur oxide is 50.0% by mass sulfur.This molecular formula could be ________.
A)SO
B)SO2
C)S2O
D)S2O4
E)either SO2 or S2O4
A)SO
B)SO2
C)S2O
D)S2O4
E)either SO2 or S2O4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
57
When the following equation is balanced,the coefficients are ________. Al(NO3)3 + Na2S → Al2S3 + NaNO3
A)2, 3, 1, 6
B)2, 1, 3, 2
C)1, 1, 1, 1
D)4, 6, 3, 2
E)2, 3, 2, 3
A)2, 3, 1, 6
B)2, 1, 3, 2
C)1, 1, 1, 1
D)4, 6, 3, 2
E)2, 3, 2, 3

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
58
How many moles of sodium carbonate contain 1.773 × 1017 carbon atoms?
A)5.890 × 10-7
B)2.945 × 10-7
C)1.473 × 10-7
D)8.836 × 10-7
E)9.817 × 10-8
A)5.890 × 10-7
B)2.945 × 10-7
C)1.473 × 10-7
D)8.836 × 10-7
E)9.817 × 10-8

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
59
How many sulfur dioxide molecules are there in 0.180 mol of sulfur dioxide?
A)1.80 × 1023
B)6.02 × 1024
C)6.02 × 1023
D)1.08 × 1024
E)1.08 × 1023
A)1.80 × 1023
B)6.02 × 1024
C)6.02 × 1023
D)1.08 × 1024
E)1.08 × 1023

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
60
When the following equation is balanced,the coefficient of Al is ________. Al (s)+ H2O (l)→ Al(OH 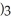 (s)+ H2 (g)
(s)+ H2 (g)
A)1
B)2
C)3
D)5
E)4
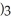 (s)+ H2 (g)
(s)+ H2 (g)A)1
B)2
C)3
D)5
E)4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
61
When the following equation is balanced,the coefficient of H3PO4 is ________. H3PO4 (aq)+ NaOH (aq)→ Na3PO4 (aq)+ H2O (l)
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
62
When the following equation is balanced,the coefficient of C3H8O3 is ________. C3H8O3 (g)+ O2 (g)→ CO2 (g)+ H2O (g)
A)1
B)2
C)3
D)7
E)5
A)1
B)2
C)3
D)7
E)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
63
When the following equation is balanced,the coefficient of water is ________. K (s)+ H2O (l)→ KOH (aq)+ H2 (g)
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
64
When the following equation is balanced,the coefficient of oxygen is ________. PbS (s)+ O2 (g)→ PbO (s)+ SO2 (g)
A)1
B)3
C)2
D)4
E)5
A)1
B)3
C)2
D)4
E)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
65
Write the balanced equation for the reaction that occurs when methanol,CH3OH (l),is burned in air. What is the coefficient of methanol in the balanced equation?
A)1
B)2
C)3
D)4
E)3/2
A)1
B)2
C)3
D)4
E)3/2

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
66
Balance the following reaction and determine the coefficient of nitric acid. N2O5 (g)+ H2O (l)→ HNO3 (aq)
A)5
B)2
C)3
D)4
E)1
A)5
B)2
C)3
D)4
E)1

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
67
When the following equation is balanced,the coefficient of dinitrogen pentoxide is ________. N2O5 (g)+ H2O (l)→ HNO3 (aq)
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck
68
When the following equation is balanced,the coefficient of sulfur dioxide is ________. PbS (s)+ O2 (g)→ PbO (s)+ SO2 (g)
A)5
B)1
C)3
D)2
E)4
A)5
B)1
C)3
D)2
E)4

Unlock Deck
Unlock for access to all 190 flashcards in this deck.
Unlock Deck
k this deck


