Deck 10: Atomic Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/143
Play
Full screen (f)
Deck 10: Atomic Physics
1
The energy of an oscillating atom in a blackbody can be any value within a certain limited range.
False
2
The peak of the radiation curve of a blackbody moves toward larger wavelength as its temperature increases.
False
3
In the Bohr model of the hydrogen atom both the energy and orbital angular momentum of the electron are quantized.
True
4
The uncertainty principle states that a particle cannot be precisely localized.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
5
A 100 eV electron and a 100 eV proton have equal de Broglie wavelengths.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
6
The highest energy level in an atom corresponds to n = 1.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
7
The peak of the radiation curve of a blackbody moves upward toward higher intensity as its temperature increases.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
8
The early Solvay Conferences had a great effect upon the development of quantum mechanics.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
9
Electrons in atoms move about the nucleus in well-defined circular orbits.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
10
A photon is quantum of electromagnetic radiation.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
11
An atom can be excited into a higher energy state by absorbing a photon.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
12
The ground state is the lowest energy level in an atom.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
13
An x-ray can be emitted when an electron undergoes an energy level transition.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
14
There is a direct correlation between the absorption and emission spectra of a particular gas.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
15
Perfect absorbers of electromagnetic radiation are also perfect emitters.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
16
In an energy level transition from the n = 2 state to the n =1 state for a hydrogen atom a photon will be emitted.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
17
The photoelectric effect occurs when electrons are emitted when light strikes the surface of a metal.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
18
In a blackbody,the energy of the oscillating atoms is quantized.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
19
The element helium was discovered by studying the Sun's spectrum.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
20
An excited atom can lose energy by emitting an electron.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
21
The chemical composition of stars can be determined from lines in their spectra.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
22
The fact that two electrons cannot occupy the same quantum state at the same time is the Heisenberg exclusion principle.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
23
Characteristic peaks occur in an x-ray spectrum of an atom because electrons in the atom make transitions to lower energy levels after being bombarded with high energy electrons.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
24
As the intensity of light is increased in a photoelectric effect experiment,the energy of the emitted electrons increases.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
25
The energy level diagram for hydrogen 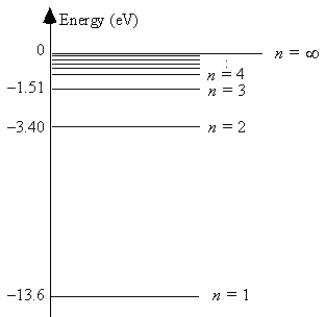
The ionization energy of hydrogen is 13.6 eV.

The ionization energy of hydrogen is 13.6 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
26
A laser can be used for very accurate measurements of distances between objects.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
27
The energy level diagram for hydrogen 
The energy of the most energetically possible photon emitted is 10.2 eV.

The energy of the most energetically possible photon emitted is 10.2 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
28
A laser emits monochromatic and coherent light.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
29
Photocopiers make use of the photoelectric effect.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
30
When the frequency of the light is increased in a photoelectric effect experiment,the energy of the emitted electrons increases.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
31
UPC price scanners at supermarket checkouts use laser holography to read the bar codes because the packages are three dimensional.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
32
The characteristic peaks in an x-ray spectrum of an atom occur because of rapid deceleration of the bombarding electrons.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
33
At any instant in time,it is possible to specify simultaneously the position and the momentum of a particle to arbitrarily high precision.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
34
When the speed of an electron is increased the de Broglie wavelength is decreased.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
35
The energy level diagram for hydrogen 
The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is 1.9 eV.

The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is 1.9 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
36
If an electron acquires more than the ionization energy,the electron will return to the ground state.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
37
Light being amplified by stimulated emission of radiation is the principle of the photoelectric effect.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
38
The energy level diagram for hydrogen 
The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is 13.6 eV.

The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is 13.6 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
39
The aurora borealis is an example of atoms and molecules in the atmosphere absorbing photons and exciting the atoms and molecules to emit spectra.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
40
Two different gases may have the same emission line spectrum.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
41
The person who first explained the blackbody spectrum was
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
42
The lowest energy level in an atom is its
A)ionization level.
B)emission level.
C)absorption level.
D)photon level.
E)ground state.
A)ionization level.
B)emission level.
C)absorption level.
D)photon level.
E)ground state.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
43
An atom can be excited into a higher energy state by
A)emitting a photon.
B)absorbing a photon.
C)undergoing the photoelectric effect.
D)decreasing its de Broglie wavelength.
E)the uncertainty principle.
A)emitting a photon.
B)absorbing a photon.
C)undergoing the photoelectric effect.
D)decreasing its de Broglie wavelength.
E)the uncertainty principle.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
44
What quantities are plotted on a blackbody radiation curve?
A)total radiated energy vs.temperature
B)radiation intensity vs.wavelength
C)wavelength of peak emission vs.temperature
D)none of the above
A)total radiated energy vs.temperature
B)radiation intensity vs.wavelength
C)wavelength of peak emission vs.temperature
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
45
What does it mean for something to be "quantized?"
A)It can have only certain discrete values.
B)It can have only integer values.
C)It can have values only between certain limits.
D)none of the above
A)It can have only certain discrete values.
B)It can have only integer values.
C)It can have values only between certain limits.
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
46
What is the symbol used for Planck's constant?
A)p
B)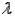
C)h
D)none of the above
A)p
B)
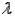
C)h
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
47
The energy associated with a photon of blue light is ________________ the energy associated with a photon of red light.
A)greater than
B)less than
C)equal to
D)unrelated to
A)greater than
B)less than
C)equal to
D)unrelated to

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
48
The fact that a particle cannot be precisely localized is the
A)photoelectric effect.
B)uncertainty principle.
C)principle of a hologram.
D)principle of a laser.
E)reason why photons are emitted.
A)photoelectric effect.
B)uncertainty principle.
C)principle of a hologram.
D)principle of a laser.
E)reason why photons are emitted.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
49
An excited atom can lose energy by
A)emitting a photon.
B)absorbing a photon.
C)undergoing the photoelectric effect.
D)increasing its de Broglie wavelength.
E)the uncertainty principle.
A)emitting a photon.
B)absorbing a photon.
C)undergoing the photoelectric effect.
D)increasing its de Broglie wavelength.
E)the uncertainty principle.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
50
Electron diffraction shows
A)that electrons behave like waves.
B)the photoelectric effect.
C)characteristic x-ray spectra.
D)that electrons in atoms have only certain allowed energies.
A)that electrons behave like waves.
B)the photoelectric effect.
C)characteristic x-ray spectra.
D)that electrons in atoms have only certain allowed energies.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
51
The quantity that is quantized in the oscillating atoms of a blackbody is
A)angular momentum.
B)energy.
C)the emission spectrum.
D)the absorption spectrum.
E)the wave function.
A)angular momentum.
B)energy.
C)the emission spectrum.
D)the absorption spectrum.
E)the wave function.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
52
What is coherent light?
A)light of a single wavelength
B)light that is all in phase
C)light that is polarized in one direction
D)none of the above
A)light of a single wavelength
B)light that is all in phase
C)light that is polarized in one direction
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
53
In the Bohr model of the hydrogen atom
A)energy is quantized.
B)the electron's angular momentum is quantized.
C)the emission spectrum is quantized.
D)the absorption spectrum is quantized.
E)all of the above
A)energy is quantized.
B)the electron's angular momentum is quantized.
C)the emission spectrum is quantized.
D)the absorption spectrum is quantized.
E)all of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
54
What is "excluded" by the Pauli exclusion principle?
A)certain values of angular momentum
B)precise values of both position and momentum
C)electrons in the same quantum state
D)none of the above
A)certain values of angular momentum
B)precise values of both position and momentum
C)electrons in the same quantum state
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
55
A three-dimensional image made using a laser is a(n)
A)emission spectrum.
B)absorption spectrum.
C)wave function.
D)hologram.
E)photoelectric effect.
A)emission spectrum.
B)absorption spectrum.
C)wave function.
D)hologram.
E)photoelectric effect.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
56
The highest energy level in an atom is its
A)ionization level.
B)emission level.
C)absorption level.
D)photon level.
E)ground state.
A)ionization level.
B)emission level.
C)absorption level.
D)photon level.
E)ground state.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
57
A quantum of electromagnetic radiation is a
A)wave function.
B)photon.
C)de Broglie wave.
D)laser.
E)hologram.
A)wave function.
B)photon.
C)de Broglie wave.
D)laser.
E)hologram.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
58
If the speed of an electron increases,its de Broglie wavelength
A)increases.
B)decreases.
C)stays the same.
D)may increase or decrease.
A)increases.
B)decreases.
C)stays the same.
D)may increase or decrease.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
59
Electrons emitted when light strikes the surface of a metal is
A)photon emission.
B)photon absorption.
C)the photoelectric effect.
D)a laser.
E)an emission spectrum.
A)photon emission.
B)photon absorption.
C)the photoelectric effect.
D)a laser.
E)an emission spectrum.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these is not involved in the operation of lasers?
A)stimulated emission
B)population inversion
C)metastable state
D)blackbody radiation
A)stimulated emission
B)population inversion
C)metastable state
D)blackbody radiation

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
61
The energy level diagram for hydrogen 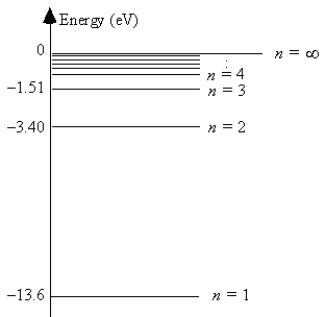
The energy of the most energetically possible photon emitted is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

The energy of the most energetically possible photon emitted is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
62
When the intensity of the light is increased in a photoelectric effect experiment,the energy of the emitted electrons
A)increases.
B)decreases.
C)stays the same.
D)becomes more quantized.
E)becomes less quantized.
A)increases.
B)decreases.
C)stays the same.
D)becomes more quantized.
E)becomes less quantized.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
63
In a laser
A)light is amplified by stimulated emission of radiation.
B)the emitted light is monochromatic.
C)the emitted light is coherent.
D)there is a population inversion of electrons in atoms.
E)all of the above
A)light is amplified by stimulated emission of radiation.
B)the emitted light is monochromatic.
C)the emitted light is coherent.
D)there is a population inversion of electrons in atoms.
E)all of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
64
The energy level diagram for hydrogen 
The ionization energy of hydrogen is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

The ionization energy of hydrogen is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
65
The person who developed the concept of a wave function was
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
66
The characteristic peaks in an x-ray spectrum of an atom occur because
A)of photon absorption.
B)of the photoelectric effect.
C)electrons in the atom make transitions to lower energy levels.
D)of the uncertainty principle.
E)of rapid deceleration of the bombarding electrons.
A)of photon absorption.
B)of the photoelectric effect.
C)electrons in the atom make transitions to lower energy levels.
D)of the uncertainty principle.
E)of rapid deceleration of the bombarding electrons.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
67
When the frequency of the light is increased in a photoelectric effect experiment,the energy of the emitted electrons
A)increases.
B)decreases.
C)stays the same.
D)becomes more quantized.
E)becomes less quantized.
A)increases.
B)decreases.
C)stays the same.
D)becomes more quantized.
E)becomes less quantized.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
68
The spectra of different elements can be distinguished because
A)they have the same spectral lines,but the lines are of different intensities.
B)they have spectral lines in different positions.
C)every element has one unique spectral line.
D)none of the above
A)they have the same spectral lines,but the lines are of different intensities.
B)they have spectral lines in different positions.
C)every element has one unique spectral line.
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
69
The person who first explained the discrete hydrogen atom spectrum was
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
70
How small an object can a scanning tunneling microscope "see?"
A)about the size of a mushroom spore
B)about the size of a living cell
C)as small as a single atom
D)as small as the nucleus of an atom
A)about the size of a mushroom spore
B)about the size of a living cell
C)as small as a single atom
D)as small as the nucleus of an atom

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
71
Lasers are used
A)to produce holograms.
B)in surgery.
C)to measure distances between objects accurately.
D)in DVD and compact disc players.
E)all of the above
A)to produce holograms.
B)in surgery.
C)to measure distances between objects accurately.
D)in DVD and compact disc players.
E)all of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
72
The continuous part of an x-ray spectrum of an atom occurs because
A)of photon absorption.
B)of the photoelectric effect.
C)electrons in the atom make transitions to lower energy levels.
D)of the uncertainty principle.
E)of rapid deceleration of the bombarding electrons.
A)of photon absorption.
B)of the photoelectric effect.
C)electrons in the atom make transitions to lower energy levels.
D)of the uncertainty principle.
E)of rapid deceleration of the bombarding electrons.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
73
The person who expounded the uncertainty principle was
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
74
The aurora borealis (northern lights)are caused by
A)sunlight reflecting from polar ice.
B)emissions from atoms and molecules in the upper atmosphere excited by electrons from the Sun.
C)thunderstorms.
D)an unknown process.
A)sunlight reflecting from polar ice.
B)emissions from atoms and molecules in the upper atmosphere excited by electrons from the Sun.
C)thunderstorms.
D)an unknown process.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
75
The energy level diagram for hydrogen 
The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

The energy of a photon emitted in a transition from the n = 3 state to the n = 2 state is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
76
What is meant by a line spectrum?
A)a spectrum which is straight
B)a spectrum made of bright lines in certain places,separated by dark gaps
C)a spectrum made by a hot filament stretched into a straight line
D)none of the above
A)a spectrum which is straight
B)a spectrum made of bright lines in certain places,separated by dark gaps
C)a spectrum made by a hot filament stretched into a straight line
D)none of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
77
The energy of a photon that ionizes a hydrogen atom from the ground state will be _________ the energy of a photon that ionizes a different hydrogen atom from the first excited state (n - 2).
A)larger than
B)smaller than
C)equal to
D)twice
A)larger than
B)smaller than
C)equal to
D)twice

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following devices make use of the photoelectric effect?
A)digital cameras
B)photocopiers
C)laser printers
D)solar cells
E)all of the above
A)digital cameras
B)photocopiers
C)laser printers
D)solar cells
E)all of the above

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
79
The energy level diagram for hydrogen 
The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

The smallest energy of an absorbed photon that will raise a hydrogen atom from its ground state to its first excited state is
A)1.5 eV.
B)1.9 eV.
C)3.4 eV.
D)10.2 eV.
E)13.6 eV.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck
80
The person who first explained the photoelectric effect was
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.
A)Max Planck.
B)Albert Einstein.
C)Neils Bohr.
D)Erwin Schrödinger.
E)Werner Heisenberg.

Unlock Deck
Unlock for access to all 143 flashcards in this deck.
Unlock Deck
k this deck


