Deck 10: Modern Atomic Theory and the Periodic Table
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 10: Modern Atomic Theory and the Periodic Table
1
The lowest possible energy level for an electron is known as
A) ground state.
B) low state.
C) basement state.
D) excited state.
A) ground state.
B) low state.
C) basement state.
D) excited state.
ground state.
2
When an electron falls from one energy level to a lower energy level,the result is the emission of
A)a beta particle.
B)quantized energy.
C)a gamma particle.
D)gamma radiation.
A)a beta particle.
B)quantized energy.
C)a gamma particle.
D)gamma radiation.
quantized energy.
3
How many orbitals are contained in the 2p sublevel?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4
3
4
Small discrete packets of energy are known as
A)excited state energy.
B)ground state energy.
C)spectra of energy.
D)quanta of energy.
A)excited state energy.
B)ground state energy.
C)spectra of energy.
D)quanta of energy.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
What is the maximum number of electrons that can occupy the first principal energy level?
A)2
B)6
C)8
D)10
A)2
B)6
C)8
D)10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which principal energy level will contain electrons with the lowest energy?
A) First
B) Second
C) Third
D) Fourth
A) First
B) Second
C) Third
D) Fourth

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
What is the maximum number of electrons that can occupy an orbital?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
What is the maximum number of electrons that can occupy the 4f sublevel?
A)2
B)6
C)10
D)14
A)2
B)6
C)10
D)14

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
In the fourth principal energy level,which sublevel contains electrons with the greatest energy?
A)4d
B)4f
C)4p
D)4s
A)4d
B)4f
C)4p
D)4s

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Which does not exist as an electron sublevel?
A)3d
B)3f
C)3p
D)3s
A)3d
B)3f
C)3p
D)3s

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
What is the maximum number of electrons that can occupy the 4s sublevel?
A)2
B)6
C)18
D)32
A)2
B)6
C)18
D)32

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which sublevel will contain electrons with the greatest energy?
A)3s
B)3p
C)3d
D)4s
A)3s
B)3p
C)3d
D)4s

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
What is the maximum number of electrons that can occupy the 3p sublevel?
A)2
B)6
C)10
D)18
A)2
B)6
C)10
D)18

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
What is the maximum number of electrons that can occupy the 3d sublevel?
A)2
B)6
C)10
D)18
A)2
B)6
C)10
D)18

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
How many orbitals are contained in the 3s sublevel?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
What is the maximum number of electrons that can occupy the third principal energy level?
A)2
B)6
C)8
D)18
A)2
B)6
C)8
D)18

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The characteristic bright line spectrum of an element is produced when electron(s)
A)move to higher energy levels.
B)fall back to lower energy levels.
C)are emitted as gamma radiation.
D)are absorbed into the nucleus.
A)move to higher energy levels.
B)fall back to lower energy levels.
C)are emitted as gamma radiation.
D)are absorbed into the nucleus.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
The distance between consecutive wave peaks is known as
A)frequency.
B)wavelength .
C)amplitude.
A)frequency.
B)wavelength .
C)amplitude.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Which scientist developed the idea that electrons exist in specific regions at various distancesfrom the nucleus?
A)Rutherford
B)Dalton
C)Thomson
D)Bohr
A)Rutherford
B)Dalton
C)Thomson
D)Bohr

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
The number of waves that pass a particular point per second is known as
A) frequency.
B) wavelength.
C) amplitude.
A) frequency.
B) wavelength.
C) amplitude.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
On the periodic table,elements in the same period contain the same number of
A)protons.
B)electrons.
C)principal energy levels in their ground state.
D)valence electrons in their ground state.
A)protons.
B)electrons.
C)principal energy levels in their ground state.
D)valence electrons in their ground state.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
On the periodic table,elements in the same group contain the same number of
A)protons.
B)electrons.
C)principal energy levels in their ground state.
D)valence electrons in their ground state.
A)protons.
B)electrons.
C)principal energy levels in their ground state.
D)valence electrons in their ground state.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Atoms of which element have the following electron configuration? 1s2 2s2 2p6 3s1
A)Sulfur
B)Strontium
C)Sodium
D)Nitrogen
A)Sulfur
B)Strontium
C)Sodium
D)Nitrogen

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
How many orbitals in an oxygen atom in the ground state contain only one electron?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
How many sublevels are occupied in an atom of magnesium in the ground state?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
How many valence electrons are present in the element with the following ground state electron configuration? 1s2 2s2 2p1
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
On the periodic table,the "transition elements" fill their last electrons in the _____ sublevel.
A)s
B)p
C)d
D)f
A)s
B)p
C)d
D)f

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
On the periodic table,elements that behave in a similar manner are found in the same
A)series.
B)cohort.
C)group.
D)period.
A)series.
B)cohort.
C)group.
D)period.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
Each vertical column on the periodic table is called a
A)series.
B)period.
C)cohort.
D)group.
A)series.
B)period.
C)cohort.
D)group.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
How many valence electrons are in an aluminum atom in the ground state?
A)1
B)2
C)3
D)13
A)1
B)2
C)3
D)13

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
How many sublevels are occupied in an atom of sulfur in the ground state?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
How many valence electrons are present in the element with the following ground state electron configuration? 1s2 2s2 2p3
A)2
B)3
C)5
D)7
A)2
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
On the periodic table,the "representative elements" are found in the
A) A Groups.
B) B Groups.
C) C Groups.
D) D Groups.
A) A Groups.
B) B Groups.
C) C Groups.
D) D Groups.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
How many orbitals are contained in the 3d sublevel?
A)2
B)5
C)7
D)10
A)2
B)5
C)7
D)10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
What is the total number of electrons in the second principal energy level of a sodium atomin the ground state?
A)2
B)6
C)8
D)11
A)2
B)6
C)8
D)11

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
How many orbitals in a nitrogen atom in the ground state contain only one electron?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
How many valence electrons are in a carbon atom in the ground state?
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
Atoms of which element have the following electron configuration?
1s2 2s2 2p3
A)Nitrogen
B)Neon
C)Nickel
D)Niobium
1s2 2s2 2p3
A)Nitrogen
B)Neon
C)Nickel
D)Niobium

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
What is the total number of electrons in the second principal energy level of a nitrogen atom in the ground state?
A)2
B)3
C)5
D)7
A)2
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Each horizontal row on the periodic table is called a
A)period
B)family
C)group
D)cohort
A)period
B)family
C)group
D)cohort

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
The number of sublevels in the third principal energy level is
A)9
B)6
C)3
D)1
A)9
B)6
C)3
D)1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
Which element is in the p-block of the periodic table?
A)Eu
B)Li
C)V
D)B
A)Eu
B)Li
C)V
D)B

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Which is not a noble gas?
A)Ra
B)Xe
C)Ar
D)Ne
A)Ra
B)Xe
C)Ar
D)Ne

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
The "alkaline earth metals" are in group
A)1A
B)2A
C)7A
D)8A
A)1A
B)2A
C)7A
D)8A

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
The electron configuration,[Ar] 4s1,is the ground state electron configuration of
A) potassium.
B) phosphorous.
C) fluorine.
D) sodium.
A) potassium.
B) phosphorous.
C) fluorine.
D) sodium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Which element is a transition element?
A)K
B)Co
C)Ca
D)Kr
A)K
B)Co
C)Ca
D)Kr

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
All of the following are used to characterize electromagnetic radiation except
A)charge
B)frequency
C)speed
D)wavelength
A)charge
B)frequency
C)speed
D)wavelength

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
What is the number of valence electrons in an alkaline earth metal?
A)1
B)2
C)7
D)12
A)1
B)2
C)7
D)12

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
The electron configuration of an atom is 1s2 2s2 2p3.The number of unpaired electrons in this atom is
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
The maximum number of electrons that can fit into a single d orbital is
A)2
B)6
C)10
D)14
A)2
B)6
C)10
D)14

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
What is the number of valence electrons in a halogen?
A)2
B)7
C)8
D)9
A)2
B)7
C)8
D)9

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following elements has the greatest number of unpaired electrons in its ground state electron configuration?
A)C
B)O
C)N
D)F
A)C
B)O
C)N
D)F

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
The "alkali" metals are in group
A)1A
B)2A
C)7A
D)8A
A)1A
B)2A
C)7A
D)8A

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
The ground state electron configuration for an atom of carbon is
A)1s2 2s2
B)1s2 2s2 2p2
C)1s2 2s2 2p4
D)1s2 2s4
A)1s2 2s2
B)1s2 2s2 2p2
C)1s2 2s2 2p4
D)1s2 2s4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
The "halogens" are in group
A)1A
B)2A
C)7A
D)8A
A)1A
B)2A
C)7A
D)8A

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
The electron configuration,[Ne] 3s2 3p4,is the ground state electron configuration of
A)sodium.
B)neon.
C)argon.
D)sulfur.
A)sodium.
B)neon.
C)argon.
D)sulfur.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
The ground state electron configuration for an atom of sodium is
A)1s2 2s2 2p6 3s1
B)1s2 2s2
C)1s2 2s2 2p6
D)1s2 2s2 2p6 3s2 3p4
A)1s2 2s2 2p6 3s1
B)1s2 2s2
C)1s2 2s2 2p6
D)1s2 2s2 2p6 3s2 3p4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
Of the orbitals shown,the one with the lowest energy is
A)2s
B)3s
C)3d
D)3p
A)2s
B)3s
C)3d
D)3p

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which element is in the d-block of the periodic table?
A)Cr
B)Am
C)Ca
D)As
A)Cr
B)Am
C)Ca
D)As

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which element is in the s-block of the periodic table?
A)Na
B)S
C)Pm
D)Mn
A)Na
B)S
C)Pm
D)Mn

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
As one descends group 2A of the periodic table,the number of valence electrons found in each element
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
The abbreviated electron configuration for arsenic is:
A)[Ar]4s24p3
B)[Ar]4s24d104p3
C)[Ar]4s23d104p3
D)[Zn]4p3
A)[Ar]4s24p3
B)[Ar]4s24d104p3
C)[Ar]4s23d104p3
D)[Zn]4p3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
The following figure shows a(an): 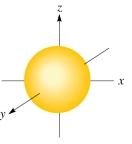
A)d orbital
B)p orbital
C)s orbital

A)d orbital
B)p orbital
C)s orbital

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
The electron configuration of an atom is 1s2 2s2 2p2.The number of occupied orbitals in this atom is
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
In an atom of chlorine,the total number of electrons in "p-type" orbitals is:
A)3
B)6
C)5
D)11
A)3
B)6
C)5
D)11

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
What is the number of orbitals in an f sublevel?
A)2
B)3
C)5
D)7
A)2
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
Which principal energy level is the first to contain f orbitals?
A)Third
B)Fourth
C)Fifth
D)Sixth
A)Third
B)Fourth
C)Fifth
D)Sixth

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Which element has a ground state electron configuration with two unpaired electrons in the 3p sublevel?
A)P
B)Mg
C)Cl
D)Si
A)P
B)Mg
C)Cl
D)Si

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
Atoms of which atomic number will have similar chemical properties to sulfur?
A)Z = 15
B)Z = 8
C)Z = 35
D)Z = 32
A)Z = 15
B)Z = 8
C)Z = 35
D)Z = 32

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
How many electrons are found in "s-type" orbitals in an atom of lithium?
A)2
B)1
C)3
D)0
A)2
B)1
C)3
D)0

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
The element in period 5 with a full d sublevel and three unpaired electrons is:
A)In
B)Sb
C)Te
D)Y
A)In
B)Sb
C)Te
D)Y

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
The following figure shows a(a): 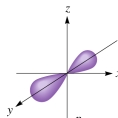
A)d orbital
B)p orbital
C)s orbital

A)d orbital
B)p orbital
C)s orbital

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Atoms of which two elements in the ground state contain the same number of valence electrons?
A)Al and Si
B)Al and B
C)Al and Zn
D)Al and Ge
A)Al and Si
B)Al and B
C)Al and Zn
D)Al and Ge

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Which atom does not have an unpaired electron in its ground state electron configuration?
A)C
B)Ca
C)P
D)Cl
A)C
B)Ca
C)P
D)Cl

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
What is the maximum number of electrons that can fit into the second principal energy level?
A)2
B)6
C)8
D)18
A)2
B)6
C)8
D)18

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
The element with an abbreviated electron configuration [Kr]5s24d6 is:
A) ruthenium
B) manganese
C) bromine
D) iron
A) ruthenium
B) manganese
C) bromine
D) iron

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Choose the correct orbital diagram for arsenic.
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
What is the maximum number of electrons that can fit into the fourth principal energy level?
A)18
B)14
C)24
D)32
A)18
B)14
C)24
D)32

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
The line spectrum of different elements provides evidence for
A)electrons traveling in orbits around the nucleus
B)the quantization of the energy of the electrons
C)the ground-state of the atom
D)the existence of a nucleus
A)electrons traveling in orbits around the nucleus
B)the quantization of the energy of the electrons
C)the ground-state of the atom
D)the existence of a nucleus

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
The following figure shows a(an): 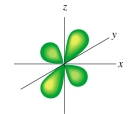
A)d orbital
B)p orbital
C)s orbital

A)d orbital
B)p orbital
C)s orbital

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


