Deck 9: Calculations From Chemical Equations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 9: Calculations From Chemical Equations
1
How many grams of molecular chlorine will be required to completely react with 0.0223 moles of sodium iodide according to the following reaction? 2NaI + Cl2  2NaCl + I2
2NaCl + I2
A)1.57 10-4 grams
10-4 grams
B)3.16 grams
C)0.0112 grams
D)0.791 grams
 2NaCl + I2
2NaCl + I2A)1.57
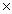 10-4 grams
10-4 gramsB)3.16 grams
C)0.0112 grams
D)0.791 grams
0.791 grams
2
How many moles of benzene (C6H6)should be completely combusted in order to produce 0.4000 moles of carbon dioxide? 2C6H6 + 15O2 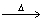 12CO2 + 6 H2O
12CO2 + 6 H2O
A)4.800 moles
B)0.2000 moles
C)2.400 moles
D)0.06667 moles
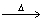 12CO2 + 6 H2O
12CO2 + 6 H2OA)4.800 moles
B)0.2000 moles
C)2.400 moles
D)0.06667 moles
0.06667 moles
3
How many grams of sodium iodide are required to react completely with 3.01 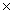 1023 molecules of chlorine? 2NaI + Cl2
1023 molecules of chlorine? 2NaI + Cl2  I2 + 2NaCl
I2 + 2NaCl
A)1.50 g
B)37.5 g
C)150.g
D)74.9 g
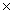 1023 molecules of chlorine? 2NaI + Cl2
1023 molecules of chlorine? 2NaI + Cl2  I2 + 2NaCl
I2 + 2NaClA)1.50 g
B)37.5 g
C)150.g
D)74.9 g
150.g
4
What mass of sodium chloride will be required to completely react with 4.00 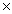 1024 units of AgNO3 and form AgCl and sodium nitrate?
1024 units of AgNO3 and form AgCl and sodium nitrate?
A)6.64 g
B)0.114 g
C)388 g
D)8.80 g
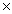 1024 units of AgNO3 and form AgCl and sodium nitrate?
1024 units of AgNO3 and form AgCl and sodium nitrate?A)6.64 g
B)0.114 g
C)388 g
D)8.80 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
How many grams of water will be required to completely react with 1.00 mole of CaO according to the following reaction? CaO + H2O  Ca(OH)2
Ca(OH)2
A)5.55 grams
B)0.0555 grams
C)18.02 grams
D)1.00 gram
 Ca(OH)2
Ca(OH)2A)5.55 grams
B)0.0555 grams
C)18.02 grams
D)1.00 gram

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
What mass of sodium hydroxide will neutralize completely 3.612 moles of HCl?
A)3.612 grams
B)144.5 grams
C)11.07 grams
D)0.09030 grams
A)3.612 grams
B)144.5 grams
C)11.07 grams
D)0.09030 grams

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
How many grams of O2 are required to produce 1.23 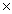 1024 molecules of water? 2H2 + O2
1024 molecules of water? 2H2 + O2  2H2O
2H2O
A)32.7 g
B)131 g
C)65.4 g
D)16.3 g
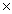 1024 molecules of water? 2H2 + O2
1024 molecules of water? 2H2 + O2  2H2O
2H2OA)32.7 g
B)131 g
C)65.4 g
D)16.3 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
How many molecules of O2 will be required to produce 1.60 moles of water? 2H2 + O2  2H2O
2H2O
A)4.82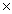 1023
1023
B)9.64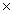 1023
1023
C)1.93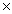 1024
1024
D)0.800
 2H2O
2H2OA)4.82
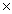 1023
1023B)9.64
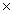 1023
1023C)1.93
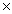 1024
1024D)0.800

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the number of moles of excess reactant that will be left-over when 50.0 g of KI react with 50.0 g of Br2: 2KI + Br2  2KBr + I2
2KBr + I2
A)both reactants are consumed completely
B)0.145 moles
C)0.0117 moles
D)0.162 moles
 2KBr + I2
2KBr + I2A)both reactants are consumed completely
B)0.145 moles
C)0.0117 moles
D)0.162 moles

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the number of moles of excess reactant that will be left-over when 56.0 g of CaCl2 react with 64.0 g of Na2SO4: CaCl2 + Na2SO4  CaSO4 + 2NaCl
CaSO4 + 2NaCl
A)0.0540 moles
B)0.0798 moles
C)0.291 moles
D)both reactants are consumed completely
 CaSO4 + 2NaCl
CaSO4 + 2NaClA)0.0540 moles
B)0.0798 moles
C)0.291 moles
D)both reactants are consumed completely

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
How many units of sodium iodide will be required to react completely with 3.01 moles of molecular bromine? 2NaI + Br2  2NaBr + I2
2NaBr + I2
A)3.63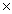 1024
1024
B)1.81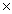 1024
1024
C)9.06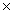 1023
1023
D)6.02
 2NaBr + I2
2NaBr + I2A)3.63
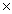 1024
1024B)1.81
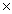 1024
1024C)9.06
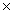 1023
1023D)6.02

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
How many moles of NH3 will be produced by the complete reaction of 1.60 moles of H2 in the following equation? N2 + 3H2 2NH3
A)1.07
B)1.60
C)2.40
D)3.20
A)1.07
B)1.60
C)2.40
D)3.20

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
How many moles of HF will be produced by the complete reaction of 1.42 moles of H2 in the following equation? H2 + F2 2HF
A)0.710
B)1.42
C)2.00
D)2.84
A)0.710
B)1.42
C)2.00
D)2.84

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
How many moles of iron(III)oxide are required to completely react with 1.40 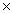 1022 atoms of carbon? Fe2O3 + 3C
1022 atoms of carbon? Fe2O3 + 3C  2Fe + 3CO
2Fe + 3CO
A)0.0232 moles
B)0.00775 moles
C)0.0697 moles
D)4.67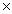 1021 moles
1021 moles
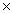 1022 atoms of carbon? Fe2O3 + 3C
1022 atoms of carbon? Fe2O3 + 3C  2Fe + 3CO
2Fe + 3COA)0.0232 moles
B)0.00775 moles
C)0.0697 moles
D)4.67
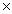 1021 moles
1021 moles
Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
How many molecules of O2 will be required to react completely with 0.600 moles of H2 in order to form water?
A)0.300
B)7.23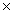 1023
1023
C)1.81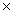 1023
1023
D)3.61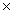 1023
1023
A)0.300
B)7.23
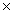 1023
1023C)1.81
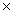 1023
1023D)3.61
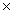 1023
1023
Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
How many moles of molecular oxygen will be needed for the complete combustion of 100.0 g of sulfur dioxide? The product of this reaction is sulfur trioxide.
A)0.7804 moles
B)1.561 moles
C)0.641 moles
D)1.281 moles
A)0.7804 moles
B)1.561 moles
C)0.641 moles
D)1.281 moles

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the number of moles of excess reactant that will be left-over when 0.350 g of HCN react with 0.500 g of O2: 4HCN + 5O2  2N2 + 4CO2 + 2H2O
2N2 + 4CO2 + 2H2O
A)0.150 moles
B)3.24 10
10
3 moles
C)4.50 10
10
4 moles
D)5.62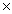 10
10
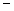
4 moles
 2N2 + 4CO2 + 2H2O
2N2 + 4CO2 + 2H2OA)0.150 moles
B)3.24
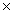 10
10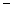
3 moles
C)4.50
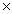 10
10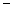
4 moles
D)5.62
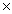 10
10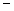
4 moles

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
Based on the following chemical equation 4HCN + 5O2  2N2 + 4CO2 + 2H2O
2N2 + 4CO2 + 2H2O
Identify the limiting reactant and the mass of N2 produced when 100.0 g of HCN react with 100.0 g of O2.
A)the limiting reactant is HCN and 25.9 g of N2 are produced
B)the limiting reactant is O2 and 35.0 g of N2 are produced
C)the limiting reactant is HCN and 51.8 g of N2 are produced
D)both reactants are consumed completely
 2N2 + 4CO2 + 2H2O
2N2 + 4CO2 + 2H2OIdentify the limiting reactant and the mass of N2 produced when 100.0 g of HCN react with 100.0 g of O2.
A)the limiting reactant is HCN and 25.9 g of N2 are produced
B)the limiting reactant is O2 and 35.0 g of N2 are produced
C)the limiting reactant is HCN and 51.8 g of N2 are produced
D)both reactants are consumed completely

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
Based on the following chemical equation 2C6H6 + 15O2 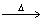 12CO2 + 6H2O
12CO2 + 6H2O
Calculate the number of moles of CO2 that will be produced when 8.90 moles of H2O are produced as well?
A)1.07 1025 moles
1025 moles
B)4.45 moles
C)17.8 moles
D)2.68 1024 moles
1024 moles
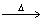 12CO2 + 6H2O
12CO2 + 6H2OCalculate the number of moles of CO2 that will be produced when 8.90 moles of H2O are produced as well?
A)1.07
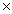 1025 moles
1025 molesB)4.45 moles
C)17.8 moles
D)2.68
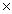 1024 moles
1024 moles
Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
What mass of sodium hydroxide will be required to neutralize completely 1.60 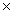 1023 molecules of HCl?
1023 molecules of HCl?
A)10.6 g
B)16.5 g
C)38.5 g
D)64.0 g
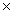 1023 molecules of HCl?
1023 molecules of HCl?A)10.6 g
B)16.5 g
C)38.5 g
D)64.0 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
How many moles of H2O are produced when 1.12 * 1023 molecule of H3PO4 are consumedin the following equation? 3Ca(OH)2 + 2H3PO4 Ca3(PO4)2 + 6H2O
A)0.0620
B)0.558
C)0.667
D)3.00
A)0.0620
B)0.558
C)0.667
D)3.00

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
How many units of K2SO4 are produced when 0.250 moles of K react completely in the following equation? 2K + MgSO4 K2SO4 + Mg
A)7.53 * 1022
B)3.01 * 1023
C)6.02 * 1023
D)1.51 * 1023
A)7.53 * 1022
B)3.01 * 1023
C)6.02 * 1023
D)1.51 * 1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
How many molecules of NH3 are produced when 3.00 moles of H2 react completely in thefollowing equation? N2 + 3H2 2NH3
A)0.667
B)1.50
C)1.20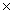 1024
1024
D)2.71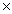 1024
1024
A)0.667
B)1.50
C)1.20
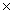 1024
1024D)2.71
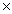 1024
1024
Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
How many moles of Zn are consumed when 1.60 * 1024 molecules of HCl react completelyin the following equation? Zn + 2HCl ZnCl2 + H2
A)1.33
B)2.66
C)3.99
D)5.32
A)1.33
B)2.66
C)3.99
D)5.32

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
How many molecules of O2 are produced when 0.500 moles of P4O10 react completely in the following equation? P4O10 4P + 5O2
A)1.21 * 1024
B)1.51 *1024
C)6.02 * 1024
D)3.01 * 1024
A)1.21 * 1024
B)1.51 *1024
C)6.02 * 1024
D)3.01 * 1024

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
How many moles of C3H8 are consumed when 1.81 * 1023 molecules of CO2 are produced in the following equation? C3H8 + 5O2 3CO2 + 4H2O
A)0.100
B)0.897
C)6.03 * 1022
D)5.43 * 1023
A)0.100
B)0.897
C)6.03 * 1022
D)5.43 * 1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of C3H6 will be consumed when 4.11 moles of CO2 are produced in the following equation? 2C3H6 + 9O2 6CO2 + 6H2O
A)1.37
B)4.11
C)6.00
D)12.3
A)1.37
B)4.11
C)6.00
D)12.3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
How many molecules of chlorine are needed to completely react with 2.00 moles of carbon according to the following equation? C + 2Cl2 CCl4
A)3.01 * 1023
B)6.02 * 1023
C)1.20 * 1024
D)2.41 *1024
A)3.01 * 1023
B)6.02 * 1023
C)1.20 * 1024
D)2.41 *1024

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
How many moles of carbon dioxide are produced when 3.00 moles of oxygen react completely in the following equation? C3H8 + 5O2 3CO2 + 9H2O
A)1.80
B)3.00
C)5.00
D)9.00
A)1.80
B)3.00
C)5.00
D)9.00

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
How many moles of H2O are produced when 1.60 * 1022 molecules of O2 react completely in the following equation? 2C2H2 + 5O2 4CO2 + 2H2O
A)0.0106
B)0.0664
C)2.41 * 1023
D)1.51 * 1024
A)0.0106
B)0.0664
C)2.41 * 1023
D)1.51 * 1024

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
How many moles of carbon dioxide are produced when 9.03 * 1023 molecules of oxygen react completely in the following equation? C3H8 + 5O2 3CO2 + 4H2O
A)0.600
B)1.67
C)0.900
D)2.50
A)0.600
B)1.67
C)0.900
D)2.50

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
How many moles of N2 will be consumed when 1.60 moles of H2 react completely in the following equation? N2 + 3H2 2NH3
A)0.533
B)1.60
C)3.20
D)4.80
A)0.533
B)1.60
C)3.20
D)4.80

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
How many molecules of O2 are consumed when 1.00 mole of N2O5 is produced in the following equation? 2N2 + 5O2 2N2O5
A)3.01 * 1024
B)1.20 * 1024
C)2.41 *1023
D)1.51 * 1024
A)3.01 * 1024
B)1.20 * 1024
C)2.41 *1023
D)1.51 * 1024

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
How many moles of CO2 are produced when 3.01 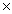 1023 molecules of C3H6 react completely in the following equation? 2C3H6 + 9O2 6CO2 + 6H2O
1023 molecules of C3H6 react completely in the following equation? 2C3H6 + 9O2 6CO2 + 6H2O
A)0.167
B)1.50
C)1.51 * 1023
D)2.01 * 1023
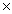 1023 molecules of C3H6 react completely in the following equation? 2C3H6 + 9O2 6CO2 + 6H2O
1023 molecules of C3H6 react completely in the following equation? 2C3H6 + 9O2 6CO2 + 6H2OA)0.167
B)1.50
C)1.51 * 1023
D)2.01 * 1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
How many moles of Al will be consumed when 0.400 moles of Al2O3 are produced in thefollowing equation? 4Al + 3O2 2Al2O3
A)0.200
B)0.400
C)0.600
D)0.800
A)0.200
B)0.400
C)0.600
D)0.800

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
How many molecules of water are produced when 3.00 moles of copper react completely in the following equation? Cu + 2H2SO4 CuSO4 + 2H2O + SO2
A)9.03 * 1023
B)3.61 * 1024
C)1.20 * 1024
D)2.71 * 1024
A)9.03 * 1023
B)3.61 * 1024
C)1.20 * 1024
D)2.71 * 1024

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
How many moles of NH3 are consumed when 1.60 * 1022 molecules of O2 react completely in the following equation? NH3 + 2O2 HNO3 + H2O
A)0.0133
B)0.608
C)1.22
D)1.83 * 1023
A)0.0133
B)0.608
C)1.22
D)1.83 * 1023

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
What mass of HCl is produced when 3.00 moles of H2 react completely in the following equation? H2 + Cl2 2HCl
A)54.7g
B)72.9g
C)109g
D)219g
A)54.7g
B)72.9g
C)109g
D)219g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
How many moles of Cl2 are consumed when 0.500 moles of AlCl3 are produced in the following equation? 2Al + 3Cl2 2AlCl3
A)0.333
B)0.500
C)0.750
D)1.00
A)0.333
B)0.500
C)0.750
D)1.00

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
How many molecules of sulfur trioxide are produced when 1.62 moles of oxygen react completely in the following equation? S8 + 12O2 8SO3
A)1.08
B)2.43
C)6.50 * 1023
D)1.46 * 1024
A)1.08
B)2.43
C)6.50 * 1023
D)1.46 * 1024

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
How many moles of CO2 are produced when 45.0g of C6H6 react completely in the following equation? 2C6H6 + 15O2 12CO2 + 6H2O
A)0.0960
B)0.289
C)3.46
D)10.4
A)0.0960
B)0.289
C)3.46
D)10.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
How many moles of N2O5 are produced when 3.00g of O2 react completely in the following equation? 2N2 + 5O2 2N2O5
A)0.0375
B)0.0750
C)0.234
D)0.469
A)0.0375
B)0.0750
C)0.234
D)0.469

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
What mass of oxygen is consumed when 54.0g of water is produced in the following equation? 2H2 + O2 2H2O
A)0.167g
B)0.667g
C)1.50g
D)47.9g
A)0.167g
B)0.667g
C)1.50g
D)47.9g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
What mass of hydrogen is produced when 0.400 moles of water react completely in the following equation? 2Na + 2H2O 2NaOH + H2
A)0.323g
B)1.62g
C)0.404g
D)2.02g
A)0.323g
B)1.62g
C)0.404g
D)2.02g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
How many moles of Al(OH)3 are produced when 50.0 g of KOH react completely in the following equation? AlCl3 + 3KOH Al(OH)3 + 3KCl
A)0.297
B)0.374
C)2.67
D)3.37
A)0.297
B)0.374
C)2.67
D)3.37

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
How many moles of carbon dioxide are produced when 12.0g of oxygen react completely in the following equation? C3H8 + 5O2 3CO2 + 4H2O
A)0.225
B)0.450
C)0.625
D)1.25
A)0.225
B)0.450
C)0.625
D)1.25

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
What mass of hydrogen is produced when 4.00 moles of zinc react completely in the following equation? Zn + 2HCl ZnCl2 + H2
A)0.505g
B)2.02g
C)6.06g
D)8.08g
A)0.505g
B)2.02g
C)6.06g
D)8.08g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
What mass of oxygen is consumed when 100.g of nitrogen react completely in the following equation? N2 + O2 2NO
A)0.112g
B)64.0g
C)100.g
D)114g
A)0.112g
B)64.0g
C)100.g
D)114g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
How many moles of CO2 are consumed when 22.0g of NH3 react completely in the following equation? CO2 + 2NH3 CO(NH2)2 + H2O
A)0.387
B)0.646
C)1.55
D)2.58
A)0.387
B)0.646
C)1.55
D)2.58

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
How many moles of oxygen are consumed when 38.0g of aluminum oxide are produced in the following equation? 4Al + 3O2 2Al2O3
A)0.248
B)0.559
C)1.50
D)3.00
A)0.248
B)0.559
C)1.50
D)3.00

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
How many moles of K2S are consumed when 14.0 g of KBr react completely in the following equation? FeBr2 + K2S 2KBr + FeS
A)0.0588
B)0.235
C)4.25
D)17.0
A)0.0588
B)0.235
C)4.25
D)17.0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
What mass of carbon monoxide is produced when 1.50 moles of oxygen react completely in the following equation? 2C + O2 2CO
A)84.0g
B)42.0g
C)28.0g
D)21.0g
A)84.0g
B)42.0g
C)28.0g
D)21.0g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
What mass of carbon dioxide is produced when 3.00 moles of oxygen react completely in the following equation? 2CO + O2 2CO2
A)88.0g
B)44.0g
C)29.3g
D)264g
A)88.0g
B)44.0g
C)29.3g
D)264g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
What mass of CO2 is produced when 50.0g of C2H4 reacts completely in the following equation? C2H4 + 3O2 2CO2 + 2H2O
A)12.3g
B)39.2g
C)49.4g
D)157g
A)12.3g
B)39.2g
C)49.4g
D)157g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
What mass of H2O is produced when 60.0g of CH4 reacts completely in the following equation? CH4 + 2O2 CO2 + 2H2O
A)2.41g
B)9.64g
C)33.7g
D)135g
A)2.41g
B)9.64g
C)33.7g
D)135g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
What mass of H2O is produced when 0.400 moles of KOH react completely in the following equation? KOH + HCl KCl + H2O
A)7.21g
B)18.0g
C)45.1g
D)90.2g
A)7.21g
B)18.0g
C)45.1g
D)90.2g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles of carbon monoxide are produced when 40.0g of oxygen react completely in the following equation? 2C + O2 2CO
A)0.625
B)1.25
C)2.50
D)80.0
A)0.625
B)1.25
C)2.50
D)80.0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
What mass of NH3 is produced when 1.20 moles of H2 react completely in the following equation? N2 + 3H2 2NH3
A)34.1g
B)20.4g
C)13.6g
D)30.7g
A)34.1g
B)20.4g
C)13.6g
D)30.7g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
What mass of H2O is produced when 12.0g of HCl react completely in the following equation?
6HCl + Fe2O3 2FeCl3 + 3H2O
A)2.97g
B)39.4g
C)27.4g
D)110.g
6HCl + Fe2O3 2FeCl3 + 3H2O
A)2.97g
B)39.4g
C)27.4g
D)110.g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
What mass of CO2 will be produced when 100.g of C8H18 react completely in the followingequation? 2C8H18 + 25O2 16CO2 + 18H2O
A)0.00249g
B)6.29g
C)308g
D)402g
A)0.00249g
B)6.29g
C)308g
D)402g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate how much ethanol,C2H5OH,will be produced from the reaction of 590.g of sugar (C6H12O6)if the reaction has a 88.5% yield. C6H12O6 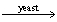 2C2H5OH + 2CO2
2C2H5OH + 2CO2
A)302 g
B)170.g
C)267 g
D)134 g
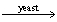 2C2H5OH + 2CO2
2C2H5OH + 2CO2A)302 g
B)170.g
C)267 g
D)134 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate the percent yield for the following reaction if 233 g of NO2 reacted and 175 g of HNO3 were produced. 3NO2 + H2O  2HNO3 + NO
2HNO3 + NO
A)75.1%
B)82.2%
C)27.4%
D)60.8%
 2HNO3 + NO
2HNO3 + NOA)75.1%
B)82.2%
C)27.4%
D)60.8%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
In a reaction to produce chlorine gas,the theoretical yield is 25.6g.What is the percent yield if the actual yield is 20.2g?
A)21.1%
B)127%
C)20.2%
D)78.9%
A)21.1%
B)127%
C)20.2%
D)78.9%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
Which is the limiting reactant when 12.0 moles of CH4 are reacted with 20.0 moles of O2 in the following equation? CH4 + 2O2 CO2 + 2H2O
A)CH4
B)O2
C)CO2
D)H2O
A)CH4
B)O2
C)CO2
D)H2O

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
Which is the limiting reactant when 3.00 moles of copper are reacted with 3.00 moles of silver nitrate in the following equation?
Cu + 2AgNO3 Cu(NO3)2 + 2Ag
A)Cu
B)AgNO3
C)Cu(NO3)2
D)Ag
Cu + 2AgNO3 Cu(NO3)2 + 2Ag
A)Cu
B)AgNO3
C)Cu(NO3)2
D)Ag

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
Which substances are in excess when 3.00 moles of Pb,1.50 moles of PbO2,and 5.00 moles of H2SO4 are reacted in the following equation? Pb + PbO2 + 2H2SO4 2PbSO4 + 2H2O
A)Pb and PbO2
B)Pb and H2SO4
C)PbO2 and H2SO4
D)PbSO4 and H2O
A)Pb and PbO2
B)Pb and H2SO4
C)PbO2 and H2SO4
D)PbSO4 and H2O

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Which substance is in excess when 6.00 moles of aluminum are reacted with 12.00 moles ofoxygen in the following equation? 4Al + 3O2 2Al2O3
A)Al
B)O2
C)Al2O3
A)Al
B)O2
C)Al2O3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the percent yield for the following reaction if 76.8 g of H2O reacted and 125 g of NO were produced. 3NO2 + H2O  2HNO3 + NO
2HNO3 + NO
A)99.0%
B)61.4%
C)60.0%
D)97.7%
 2HNO3 + NO
2HNO3 + NOA)99.0%
B)61.4%
C)60.0%
D)97.7%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Which is the limiting reactant when 3.00 moles of calcium are reacted with 8.00 moles of water in the following equation? Ca + 2H2O Ca(OH)2 + H2
A)Ca
B)H2O
C)Ca(OH)2
D)H2
A)Ca
B)H2O
C)Ca(OH)2
D)H2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
Which substance is in excess when 2.00 moles of cobalt are reacted with 1.50 moles of lead(II)chloride in the following equation? Co + PbCl2 CoCl2 + Pb
A)Co
B)PbCl2
C)CoCl2
D)Pb
A)Co
B)PbCl2
C)CoCl2
D)Pb

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
What mass of sulfur dioxide is produced when 18.0g of sulfur react completely in the following equation? S + O2 SO2
A)0.00876g
B)0.0278g
C)36.0g
D)114g
A)0.00876g
B)0.0278g
C)36.0g
D)114g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
In a reaction to produce iron the theoretical yield is 340 kg.What is the percent yield if the actual yield is 300.kg?
A)13.3%
B)88.2%
C)11.8%
D)113%
A)13.3%
B)88.2%
C)11.8%
D)113%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Which substance is in excess when 1.50 moles of aluminum bromide are reacted with 2.50 moles of barium hydroxide in the following equation? 2AlBr3 + 3Ba(OH)2 3BaBr2 + 2Al(OH)3
A)BaBr2
B)Al(OH)3
C)Ba(OH)2
D)AlBr3
A)BaBr2
B)Al(OH)3
C)Ba(OH)2
D)AlBr3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
What is the percent yield for a reaction in which 342g of a product is actually produced when the theoretical yield is 400.g?
A)10.5%
B)80.0%
C)97.6%
D)85.5%
A)10.5%
B)80.0%
C)97.6%
D)85.5%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
Calculate how much acetylene (C2H2)can be produced from 358 g of H2O and an excess of CaC2 if the percent yield for this reaction is 94.5%. CaC2 + 2H2O  C2H2 + Ca(OH)2
C2H2 + Ca(OH)2
A)338 g
B)489 g
C)244 g
D)259 g
 C2H2 + Ca(OH)2
C2H2 + Ca(OH)2A)338 g
B)489 g
C)244 g
D)259 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
In a reaction to produce sulfuric acid,the theoretical yield is 300.g.What is the percent yield if the actual yield is 280.g?
A)6.67%
B)7.14%
C)93.3%
D)107%
A)6.67%
B)7.14%
C)93.3%
D)107%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
What is the percent yield in a reaction in which 1.038g of a product is actually produced when the theoretical yield is 1.132g?
A)8.304%
B)109.1%
C)89.16%
D)91.70%
A)8.304%
B)109.1%
C)89.16%
D)91.70%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
Which is the limiting reactant when 3.00 moles of MnO2 are reacted with 10.0 moles of HClin the following equation? MnO2 + 4HCl MnCl2 + 2H2O + Cl2
A)MnO2
B)HCl
C)MnCl2
D)H2O
A)MnO2
B)HCl
C)MnCl2
D)H2O

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
In a reaction to produce ammonia,the theoretical yield is 420.g.What is the percent yield if the actual yield is 350.g?
A)83.3%
B)20.0%
C)16.7%
D)120.%
A)83.3%
B)20.0%
C)16.7%
D)120.%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
What is the percent yield for a reaction in which 24.6g of a product is actually produced when the theoretical yield is 30.0g?
A)82.0%
B)122%
C)21.0%
D)18.0%
A)82.0%
B)122%
C)21.0%
D)18.0%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


