Deck 12: The Gaseous State of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 12: The Gaseous State of Matter
1
A pressure of 340.mm Hg is equal to
A)2.24 torr
B)0.448 torr
C)340.torr
D)1.25 torr
A)2.24 torr
B)0.448 torr
C)340.torr
D)1.25 torr
340.torr
2
A pressure of 3.00 atm is equal to
A)819 mm Hg
B)3000 mm Hg
C)2280 mm Hg
D)253 mm Hg
A)819 mm Hg
B)3000 mm Hg
C)2280 mm Hg
D)253 mm Hg
2280 mm Hg
3
Which of the following gases would have the greatest kinetic energy at 300 K?
A)N2
B)NH3
C)Ar
D)All of them would have the same kinetic energy
A)N2
B)NH3
C)Ar
D)All of them would have the same kinetic energy
All of them would have the same kinetic energy
4
Which gas will diffuse most rapidly?
A)CO2
B)N2
C)He
D)Cl2
A)CO2
B)N2
C)He
D)Cl2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
A pressure of 1030 torr is equal to
A)0.738 atm
B)0.265 atm
C)1.36 atm
D)3.77 atm
A)0.738 atm
B)0.265 atm
C)1.36 atm
D)3.77 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
A 120.mL sample of a gas is at a pressure of 1.50 atm.If the temperature remains constant, what will be its volume at 3.50 atm of pressure?
A)280.mL
B)22.9 mL
C)120.mL
D)51.4 mL
A)280.mL
B)22.9 mL
C)120.mL
D)51.4 mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Under which set of conditions will a real gas be most likely to act as an ideal gas?
A)High temperature and high pressure
B)High temperature and low pressure
C)Low temperature and high pressure
D)Low temperature and low pressure
A)High temperature and high pressure
B)High temperature and low pressure
C)Low temperature and high pressure
D)Low temperature and low pressure

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
The following figure represents one mole of an ideal gas in a container fit with a movable piston. 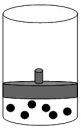 Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
A)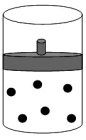
B)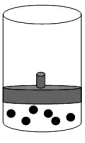
C)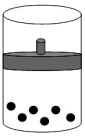
D)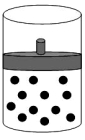
 Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?
Which figure shows the change,if any,that would take place if the Kelvin temperature is doubled under constant pressure?A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
As the number of molecules in a gas sample increases,temperature and volume remaining constant,the pressure exerted by the gas
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
A 4.00 L sample of a gas is at a pressure of 2.00 atm.If the temperature remains constant, what will be its volume at 0.500 atm of pressure?
A)1.00 L
B)16.0 L
C)0.250 L
D)4.00 L
A)1.00 L
B)16.0 L
C)0.250 L
D)4.00 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
As the temperature of a gas sample increases,the number of molecules and volume remaining constant,the pressure exerted by the gas
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
A hot air balloon is filled to a volume of 44.5 L at 758 torr.What will be the volume of the balloon if the pressure decreases to 748 torr under constant temperature?
A)45.1 L
B)43.9 L
C)44.5 L
D)49.0 L
A)45.1 L
B)43.9 L
C)44.5 L
D)49.0 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
Which gas would have the lowest velocity at 280 K?
A)Ar
B)Ne
C)H2
D)CO
A)Ar
B)Ne
C)H2
D)CO

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
A sample of a gas is held at constant temperature.If the number of moles of gas in the sample is doubled while the pressure is halved,what will happen to the volume of the gas sample?
A)It will increase.
B)It will decrease.
C)It will not change.
A)It will increase.
B)It will decrease.
C)It will not change.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
A pressure of 3240 torr is equal to
A)0.235 mm Hg
B)11.9 mm Hg
C)4.26 mm Hg
D)3240 mm Hg
A)0.235 mm Hg
B)11.9 mm Hg
C)4.26 mm Hg
D)3240 mm Hg

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
As the pressure of a sample of gas is increased at constant temperature,the volume of the gaswill
A)increases.
B)decreases.
C)remain the same.
A)increases.
B)decreases.
C)remain the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
The following figure shows 1 mol of an ideal gas inside a container with a movable piston.  If we add two additional moles of gas under constant temperature and pressure,select the alternative that represents the change,if any,that will take place.
If we add two additional moles of gas under constant temperature and pressure,select the alternative that represents the change,if any,that will take place.
A)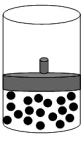
B)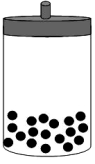
C)
D)
 If we add two additional moles of gas under constant temperature and pressure,select the alternative that represents the change,if any,that will take place.
If we add two additional moles of gas under constant temperature and pressure,select the alternative that represents the change,if any,that will take place.A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Under which set of conditions will a real gas be least likely to act as an ideal gas?
A)High temperature and high pressure
B)High temperature and low pressure
C)Low temperature and high pressure
D)Low temperature and low pressure
A)High temperature and high pressure
B)High temperature and low pressure
C)Low temperature and high pressure
D)Low temperature and low pressure

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
A 3.00 L sample of a gas at a pressure of 4.00 atm is compressed to 2.00 L at a constant temperature.What is the pressure of the gas?
A)2.00 atm
B)6.00 atm
C)12.0 atm
D)24.0 atm
A)2.00 atm
B)6.00 atm
C)12.0 atm
D)24.0 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
A pressure of 414 mm Hg is equal to
A)0.545 atm
B)0.659 atm
C)1.52 atm
D)1.84 atm
A)0.545 atm
B)0.659 atm
C)1.52 atm
D)1.84 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
At STP,3.00 L of nitrogen gas contains the same number of molecules as
A)1.00 L of nitrogen gas
B)2.00 L of oxygen gas
C)3.00 L of chlorine gas
D)4.00 L of hydrogen gas
A)1.00 L of nitrogen gas
B)2.00 L of oxygen gas
C)3.00 L of chlorine gas
D)4.00 L of hydrogen gas

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
A sample of gas has a volume of 3.40 L at 10.0 °C.What will be its volume at 100.0°C, pressure remaining constant?
A)0.340 L
B)4.48 L
C)34.0 L
D)2.58 L
A)0.340 L
B)4.48 L
C)34.0 L
D)2.58 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
At STP,which sample contains twice the number of molecules found in 4.00 L of hydrogen gas?
A)1.00 L of He
B)2.00 L of He
C)4.00 L of He
D)8.00 L of He
A)1.00 L of He
B)2.00 L of He
C)4.00 L of He
D)8.00 L of He

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
A sample of gas has a volume of 200.mL at 20.0 ° C.What will be its volume at 40.0 °C, pressure remaining constant?
A)18.8 mL
B)214 mL
C)100.mL
D)400.mL
A)18.8 mL
B)214 mL
C)100.mL
D)400.mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
A mixture of gases consists of helium at a partial pressure of 400.torr,neon at a partial pressure of 300.torr,and argon at a partial pressure of 200.torr.What is the total pressure of this mixture of gases?
A)300.torr
B)760.torr
C)900.torr
D)1000 torr
A)300.torr
B)760.torr
C)900.torr
D)1000 torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
A sample of gas has a volume of 8.00 L at 20.0 ° C and 700.torr.What will be its volume atSTP?
A)1.20 L
B)9.32 L
C)53.2 L
D)6.87 L
A)1.20 L
B)9.32 L
C)53.2 L
D)6.87 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of gas is in a cylinder of fixed volume.At 30.0 °C its internal pressure is 3200. torr.What will be its pressure at 12.0 ° C?
A)8000.torr
B)1280.torr
C)3400.torr
D)3010.torr
A)8000.torr
B)1280.torr
C)3400.torr
D)3010.torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
A sample of oxygen is collected over water at 22 ° C and 762 torr.What is the partial pressure of the dry oxygen? The vapor pressure of water at 22°C is 19.8 torr.
A)742 torr
B)782 torr
C)784 torr
D)750.torr
A)742 torr
B)782 torr
C)784 torr
D)750.torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
A sample of gas has a volume of 400.mL at STP.What will be its volume at 20.0 ° C and700.torr?
A)466 mL
B)343 mL
C)60.1 mL
D)2660 mL
A)466 mL
B)343 mL
C)60.1 mL
D)2660 mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
The volume of a gas must always decrease when
A)temperature increases and pressure increases.
B)temperature increases and pressure decreases.
C)temperature decreases and pressure increases.
D)temperature decreases and pressure decreases.
A)temperature increases and pressure increases.
B)temperature increases and pressure decreases.
C)temperature decreases and pressure increases.
D)temperature decreases and pressure decreases.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
A 400.mL sample of hydrogen is collected over water at 23.0 ° C and 758.4 torr.What isthe volume of the dry hydrogen gas at 23.0 ° C and 758.4 torr? The vapor pressure of water at 23.0 ° C is 21.1 torr.
A)144 mL
B)389 mL
C)411 mL
D)14400 mL
A)144 mL
B)389 mL
C)411 mL
D)14400 mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
A sample of gas has a volume of 1380 mL at a temperature of 20.0 ° C.What will be its volume at 0.00°C,pressure remaining constant?
A)0.00 mL
B)1290 mL
C)1380 mL
D)1480 mL
A)0.00 mL
B)1290 mL
C)1380 mL
D)1480 mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
What is the volume of 0.132 moles of carbon dioxide gas at STP?
A)2.96 L
B)5.81 L
C)170.L
D)333 L
A)2.96 L
B)5.81 L
C)170.L
D)333 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
A sample of gas is in a cylinder of fixed volume.At 20.0 °C its internal pressure is 3.42 atm.What will be its pressure at 120.0°C?
A)2.55 atm
B)4.59 atm
C)0.570 atm
D)20.5 atm
A)2.55 atm
B)4.59 atm
C)0.570 atm
D)20.5 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
What is the volume of 3.00 moles of neon gas at STP?
A)6.73 L
B)7.47 L
C)60.6 L
D)67.2 L
A)6.73 L
B)7.47 L
C)60.6 L
D)67.2 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
As the temperature of a sample of gas is increased at constant pressure,its volume will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
A sample of gas is in a cylinder of fixed volume.As the temperature of the sampleincreases,its internal pressure will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of gas is in a cylinder of fixed volume.At 20.0 ° C its pressure is 4.00 atm.At what temperature will its pressure be 8.00 atm?
A)10.0 ° C
B)40.0 ° C
C)-127°C
D)313 ° C
A)10.0 ° C
B)40.0 ° C
C)-127°C
D)313 ° C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
The volume of a gas must always increase when
A)temperature increases and pressure increases.
B)temperature increases and pressure decreases.
C)temperature decreases and pressure increases.
D)temperature decreases and pressure decreases.
A)temperature increases and pressure increases.
B)temperature increases and pressure decreases.
C)temperature decreases and pressure increases.
D)temperature decreases and pressure decreases.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of gas has a volume of 850.mL at 23.0 ° C and 1.10 atm.The temperature is increased to 33.0 ° C,at what pressure will its volume be 900.mL?
A)1.20 atm
B)1.49 atm
C)1.07 atm
D)0.812 atm
A)1.20 atm
B)1.49 atm
C)1.07 atm
D)0.812 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
How many moles are present in 10.0 L of nitrogen gas at STP?
A)2.24 moles
B)2.80 moles
C)0.446 moles
D)224 moles
A)2.24 moles
B)2.80 moles
C)0.446 moles
D)224 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
How many moles are present in 300.L of ammonia gas at STP?
A)0.0568 moles
B)13.4 moles
C)0.0747 moles
D)17.6 moles
A)0.0568 moles
B)13.4 moles
C)0.0747 moles
D)17.6 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
What is the molar mass of a gas if 18.0 L of the gas at STP has a mass of 41.6 grams?
A)51.8 g
B)9.69 g
C)33.4 g
D)1.86 g
A)51.8 g
B)9.69 g
C)33.4 g
D)1.86 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
What is the molar mass of a gas if 30.0 L of the gas at STP has a mass of 16.0 grams?
A)2.05 g
B)11.9 g
C)21.4 g
D)42.0 g
A)2.05 g
B)11.9 g
C)21.4 g
D)42.0 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
What is the molar mass of a gas if 40.0 L of the gas has a mass of 36.0 g at 740.torr and 30.0 ° C?
A)333 g
B)33.1 g
C)23.0 g
D)56.0 g
A)333 g
B)33.1 g
C)23.0 g
D)56.0 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
What is the volume of 1.40 moles of chlorine gas at STP?
A)31.4 L
B)16.0 L
C)0.316 L
D)3.17 L
A)31.4 L
B)16.0 L
C)0.316 L
D)3.17 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
What is the molar mass of a gas if 12.0 L of the gas at STP has a mass of 16.0 grams?
A)8.57 g
B)22.4 g
C)16.8 g
D)29.9 g
A)8.57 g
B)22.4 g
C)16.8 g
D)29.9 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
What is the density of nitrogen gas at STP?
A)0.625 g/L
B)0.799 g/L
C)1.60 g/L
D)1.25 g/L
A)0.625 g/L
B)0.799 g/L
C)1.60 g/L
D)1.25 g/L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
At what temperature would 0.600 mole of a gas at 700.torr have a volume of 22.6 L?
A)150. ° C
B)225 ° C
C)423 ° C
D)498 ° C
A)150. ° C
B)225 ° C
C)423 ° C
D)498 ° C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
What is the molar mass of a gas if 26.0 L of the gas has a mass of 43.6 g at 1.10 atm and 10.0 ° C?
A)35.4 g
B)0.799 g
C)17.9 g
D)0.0282 g
A)35.4 g
B)0.799 g
C)17.9 g
D)0.0282 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
What volume of carbon dioxide gas is produced when 3.00 L of oxygen gas react with 7.5 L of carbon monoxide in the following equation?
2 CO(g)+ O2(g) 2 CO2(g)
A)6.00 L
B)10.5 L
C)6.75 L
D)3.00 L
2 CO(g)+ O2(g) 2 CO2(g)
A)6.00 L
B)10.5 L
C)6.75 L
D)3.00 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
What is the volume of 1.40 moles of nitrogen gas at 20.0 ° C and 770.torr?
A)33.2 L
B)2.27 L
C)0.0437 L
D)405 L
A)33.2 L
B)2.27 L
C)0.0437 L
D)405 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
How many moles are present in 30.0 L of helium gas at STP?
A)0.179 moles
B)0.747 moles
C)1.34 moles
D)7.47 moles
A)0.179 moles
B)0.747 moles
C)1.34 moles
D)7.47 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molar mass of a gas if 16.0 L of the gas has a mass of 19.0 g at 765 torr and 20.0 °C?
A)0.0352 g
B)28.4 g
C)26.8 g
D)1.94 g
A)0.0352 g
B)28.4 g
C)26.8 g
D)1.94 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
What volume of oxygen is consumed when 3.00 moles of carbon monoxide is produced in the following equation? 2 C(s)+ O2(g) 2 CO(g)
A)1.50 L
B)33.6 L
C)67.2 L
D)22.4 L
A)1.50 L
B)33.6 L
C)67.2 L
D)22.4 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
At which pressure would a 2.60 mole sample of gas at 20.0 ° C have a volume of 25.0 L?
A)0.171 atm
B)15.8 atm
C)2.08 atm
D)2.50 atm
A)0.171 atm
B)15.8 atm
C)2.08 atm
D)2.50 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
What is the density of carbon dioxide gas at STP?
A)1.96 g/L
B)0.509 g/L
C)2.40 g/L
D)1.00 g/L
A)1.96 g/L
B)0.509 g/L
C)2.40 g/L
D)1.00 g/L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is not an assumption under KMT?
A)Gas particles have no attraction for one another.
B)During a collision,energy is lost by the gas particles and later is regained.
C)Gases at the same temperature have the same average kinetic energy.
D)Gas particles move in straight lines in all directions.
A)Gas particles have no attraction for one another.
B)During a collision,energy is lost by the gas particles and later is regained.
C)Gases at the same temperature have the same average kinetic energy.
D)Gas particles move in straight lines in all directions.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
What volume of ammonia is produced when 0.500 mole of nitrogen reacts completely in the following equation?
N2(g)+ 3 H2(g) 2NH3(g)
A)1.00 L
B)22.4 L
C)44.8 L
D)11.2 L
N2(g)+ 3 H2(g) 2NH3(g)
A)1.00 L
B)22.4 L
C)44.8 L
D)11.2 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
How many grams of oxygen are consumed when 18.0 L of CH4 gas react completely in the following equation? CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(g)
A)51.4 grams
B)79.7 grams
C)576 grams
D)1.15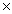 103 grams
103 grams
A)51.4 grams
B)79.7 grams
C)576 grams
D)1.15
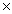 103 grams
103 grams
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
A 64.0 g sample of oxygen contains the same number of molecules as
A)44.8 L of nitrogen
B)14.01 g of nitrogen
C)24.8 L of nitrogen
D)28.02 g of nitrogen
A)44.8 L of nitrogen
B)14.01 g of nitrogen
C)24.8 L of nitrogen
D)28.02 g of nitrogen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Look at the apparatus shown below.Inside each chamber there is a sample of a gas at the specified volume and pressure.Once the stopcock is opened,what will be the total pressure inside the system.The whole process is done under constant temperature. 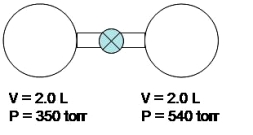
A)890 torr
B)445 torr
C)190 torr
D)223 torr

A)890 torr
B)445 torr
C)190 torr
D)223 torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Which gas is most dense at STP?
A)Helium
B)Nitrogen
C)Oxygen
D)Hydrogen
A)Helium
B)Nitrogen
C)Oxygen
D)Hydrogen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
What is the volume occupied by 25.0 g of oxygen gas at a pressure of 733 mm Hg and a temperature of 30.5°C?
A)20.2 L
B)2.03 L
C)26.5 L
D)6.46 L
A)20.2 L
B)2.03 L
C)26.5 L
D)6.46 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
Calculate the density of argon gas at STP.
A)2.24 g/L
B)1.36 g/L
C)1.78 g/L
D)1.63 g/L
A)2.24 g/L
B)1.36 g/L
C)1.78 g/L
D)1.63 g/L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
An 11.2 L sample of nitrogen contains the same number of molecules as
A)8.00 g of oxygen
B)16.0 g of oxygen
C)22.4 L of oxygen
D)5.60 L of oxygen
A)8.00 g of oxygen
B)16.0 g of oxygen
C)22.4 L of oxygen
D)5.60 L of oxygen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Which gas has approximately the same density as carbon dioxide gas at STP?
A)CH4
B)C2H6
C)C3H8
D)C4H10
A)CH4
B)C2H6
C)C3H8
D)C4H10

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
A mixture containing 5.00 g of H2(g)and 10.0 g of O2(g)is allowed to react in a 15.0 L rigid container at a constant temperature of 25.0°C. 2H2(g)+ O2(g)  2H2O(l)
2H2O(l)
Once the reaction is complete,what will be the pressure of the excess gas inside the container?
A)1.51 atm
B)4.04 atm
C)3.53 atm
D)3.02 atm
 2H2O(l)
2H2O(l)Once the reaction is complete,what will be the pressure of the excess gas inside the container?
A)1.51 atm
B)4.04 atm
C)3.53 atm
D)3.02 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
How many moles of hydrogen are consumed when 40.0 L of hydrogen chloride gas are produced in the following equation?
H2(g)+ Cl2(g) 2 HCl(g)
A)0.446 moles
B)0.893 moles
C)1.79 moles
D)3.57 moles
H2(g)+ Cl2(g) 2 HCl(g)
A)0.446 moles
B)0.893 moles
C)1.79 moles
D)3.57 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
A mixture of 10.0 g of Ne and 10.0 g of Ar was kept in a 1.00 L container at 765 torr.Calculate the partial pressure of Ar inside the container.
A)508 torr
B)257 torr
C)614 torr
D)125 torr
A)508 torr
B)257 torr
C)614 torr
D)125 torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
When the following reaction took place at 23.8 °C and a pressure of 758 torr,3.90 L of O2(g)were collected.What mass of KClO3 decomposed? 2KClO3(s)  2KCl(s)+ 3O2(g)
2KCl(s)+ 3O2(g)
A)163 g
B)19.6 g
C)13.0 g
D)39.1 g
 2KCl(s)+ 3O2(g)
2KCl(s)+ 3O2(g)A)163 g
B)19.6 g
C)13.0 g
D)39.1 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
What mass of water is produced when 10.0 L of oxygen react completely in the following equation? 2 H2(g)+ O2(g) 2 H2O(l)
A)4.02 g
B)16.1 g
C)20.1 g
D)80.7 g
A)4.02 g
B)16.1 g
C)20.1 g
D)80.7 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
A gas mixture containing N2 and O2 was kept inside a 2.00 L container at a temperature of 23.0°C and a total pressure of 1.00 atm.The partial pressure of oxygen was 0.722 atm.How many grams of nitrogen were present in the gas mixture?
A)2.31 g
B)0.577 g
C)0.641 g
D)1.90 g
A)2.31 g
B)0.577 g
C)0.641 g
D)1.90 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
What mass of water is produced when 10.0 L of oxygen react completely in the following equation? 2 C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(g)
A)6.90 g
B)21.0 g
C)9.39 g
D)34.6 g
A)6.90 g
B)21.0 g
C)9.39 g
D)34.6 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
What volume of chlorine is consumed when 12.0 g of HCl is produced in the following equation? H2(g)+ Cl2(g) 2 HCl(g)
A)3.69 L
B)163 L
C)14.7 L
D)40.8 L
A)3.69 L
B)163 L
C)14.7 L
D)40.8 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
A sample of an ideal gas is kept in a rigid container while the Kelvin temperature is doubled.What will happen to the pressure of the gas?
A)The pressure will double.
B)The pressure will be halved.
C)The pressure will remain constant.
D)The pressure will increase by a factor of 1.5.
A)The pressure will double.
B)The pressure will be halved.
C)The pressure will remain constant.
D)The pressure will increase by a factor of 1.5.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
How many moles of oxygen are consumed when 100.L of dinitrogen pentoxide are produced in the following equation?
2 N2(g)+ 5 O2(g) 2 N2O5(g)
A)0.560 moles
B)1.79 moles
C)0.0896 moles
D)11.2 moles
2 N2(g)+ 5 O2(g) 2 N2O5(g)
A)0.560 moles
B)1.79 moles
C)0.0896 moles
D)11.2 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
What mass of helium would be contained within a 1.8 L balloon at a pressure of 766 torr and a temperature of 24.5°C?
A)3.61 g
B)0.297 g
C)226 g
D)0.594 g
A)3.61 g
B)0.297 g
C)226 g
D)0.594 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
What volume of dinitrogen pentoxide gas is produced when 500.mL of oxygen react completely in the following equation? 2 N2(g)+ 5 O2(g) 2 N2O5(g)
A)500.mL
B)400.mL
C)1250 mL
D)200.mL
A)500.mL
B)400.mL
C)1250 mL
D)200.mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
What volume of sulfur dioxide gas will be consumed when 12.0 L of oxygen is consumed in the following equation? 2 SO2(g)+ O2(g) 2 SO3(g)
A)6.00 L
B)12.0 L
C)24.0 L
D)60.0 L
A)6.00 L
B)12.0 L
C)24.0 L
D)60.0 L

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


