Deck 13: Liquids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 13: Liquids
1
The following table lists normal boiling points for different substances: 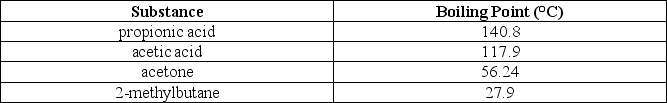 Which of the following alternatives is true?
Which of the following alternatives is true?
A)The vapor pressure of propionic acid is the highest of all the substances listed under these conditions.
B)The molecules of acetone experience the weakest interactions compared to any other substance listed.
C)The molecules of propionic acid experience the strongest interactions compared to any other substance listed.
D)Alternatives A and C are correct.
 Which of the following alternatives is true?
Which of the following alternatives is true?A)The vapor pressure of propionic acid is the highest of all the substances listed under these conditions.
B)The molecules of acetone experience the weakest interactions compared to any other substance listed.
C)The molecules of propionic acid experience the strongest interactions compared to any other substance listed.
D)Alternatives A and C are correct.
The molecules of propionic acid experience the strongest interactions compared to any other substance listed.
2
Which phase change corresponds to condensation?
A)Liquid to gas
B)Solid to liquid
C)Solid to gas
D)Gas to liquid
A)Liquid to gas
B)Solid to liquid
C)Solid to gas
D)Gas to liquid
Gas to liquid
3
As the volatility of a liquid increases,its equilibrium vapor pressure
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.
increases.
4
What is the vapor pressure of water at 100.°C?
A)0 torr
B)100 torr
C)373 torr
D)760 torr
A)0 torr
B)100 torr
C)373 torr
D)760 torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
The following table lists some compounds and their respective surface tensions at 25°C: 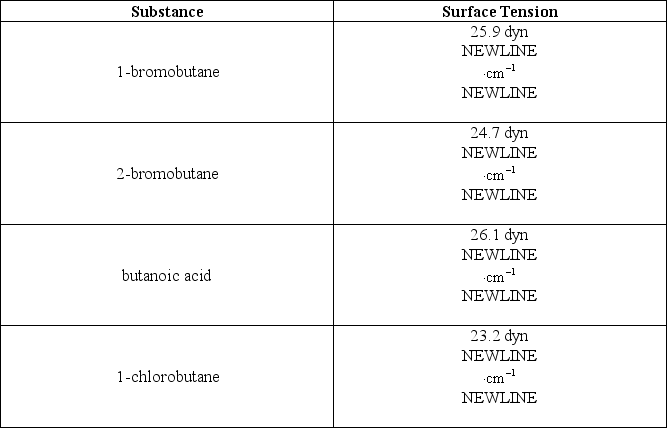 Which of the following alternatives is false?
Which of the following alternatives is false?
A)The attractive forces between molecules of butanoic acid are stronger than those between molecules of 1-chlorobutane.
B)The attractive forces between molecules of 1-chlorobutane are stronger than those between molecules of 1-bromobutane.
C)Molecules of 1-chlorobutane experience the least resistance to increase their surface area compared to any of the other compounds.
D)The compounds 1-chlorobutane,2-bromobutane,1-bromobutane,and butanoic acid are arranged in order of increasing strength of their intermolecular forces.
 Which of the following alternatives is false?
Which of the following alternatives is false?A)The attractive forces between molecules of butanoic acid are stronger than those between molecules of 1-chlorobutane.
B)The attractive forces between molecules of 1-chlorobutane are stronger than those between molecules of 1-bromobutane.
C)Molecules of 1-chlorobutane experience the least resistance to increase their surface area compared to any of the other compounds.
D)The compounds 1-chlorobutane,2-bromobutane,1-bromobutane,and butanoic acid are arranged in order of increasing strength of their intermolecular forces.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
In a system at equilibrium between the liquid and gas phases
A)the rate at which particles change from gas to liquid exceeds the rate at which they change from liquid to gas.
B)the rate at which particles change from liquid to gas exceeds the rate at which they change from gas to liquid.
C)the rate at which particles change from gas to liquid equals the rate at which they change from liquid to gas.
D)particles stop changing phase.
A)the rate at which particles change from gas to liquid exceeds the rate at which they change from liquid to gas.
B)the rate at which particles change from liquid to gas exceeds the rate at which they change from gas to liquid.
C)the rate at which particles change from gas to liquid equals the rate at which they change from liquid to gas.
D)particles stop changing phase.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Which has the highest vapor pressure?
A)25 mL of water at 283 K
B)10 mL of water at 298 K
C)50 mL of water at 293 K
D)5 mL of water at 323 K
A)25 mL of water at 283 K
B)10 mL of water at 298 K
C)50 mL of water at 293 K
D)5 mL of water at 323 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
Which phase change corresponds to sublimation?
A)Solid to gas
B)Liquid to gas
C)Gas to liquid
D)Solid to liquid
A)Solid to gas
B)Liquid to gas
C)Gas to liquid
D)Solid to liquid

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
The vapor pressure of a liquid is the pressure,at equilibrium,of its
A)solid above its liquid.
B)liquid above its solid.
C)gas above its liquid.
D)liquid above its gas.
A)solid above its liquid.
B)liquid above its solid.
C)gas above its liquid.
D)liquid above its gas.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
As the attractive forces between the molecules of a liquid increase,its volatility
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
Which phase change corresponds to evaporation?
A)Solid to liquid
B)Solid to gas
C)Liquid to gas
D)Liquid to solid
A)Solid to liquid
B)Solid to gas
C)Liquid to gas
D)Liquid to solid

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
As the rate of evaporation of a liquid increases,its equilibrium vapor pressure
A)Increases
B)Decreases
C)Remains the same
A)Increases
B)Decreases
C)Remains the same

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
Which has the lowest vapor pressure?
A)25 mL of water at 283 K
B)10 mL of water at 298 K
C)50 mL of water at 293 K
D)5 mL of water at 323 K
A)25 mL of water at 283 K
B)10 mL of water at 298 K
C)50 mL of water at 293 K
D)5 mL of water at 323 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
As the attractive forces between the molecules of a liquid increase,its equilibrium vapor pressure
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Use the following graph to select the boiling point of ethyl alcohol at 400 torr. 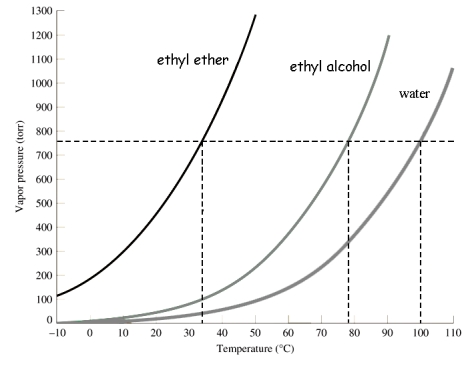
A)about 35°C
B)about 78°C
C)about 20°C
D)about 62°C

A)about 35°C
B)about 78°C
C)about 20°C
D)about 62°C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
What is the boiling point of water at one atmosphere of pressure?
A)0 K
B)100 K
C)273 K
D)373 K
A)0 K
B)100 K
C)273 K
D)373 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
The boiling point temperature of a liquid is the temperature at which its vapor pressure
A)is less than the external pressure.
B)is greater than the external pressure.
C)is equal to the external pressure.
A)is less than the external pressure.
B)is greater than the external pressure.
C)is equal to the external pressure.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
As the attractive forces between the molecules of a liquid increase,its surface tension
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
Which segment in the following figure corresponds to melting? 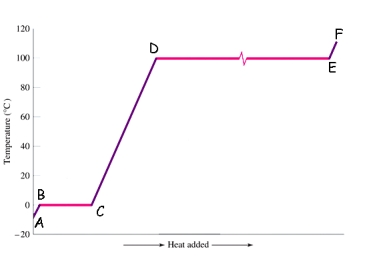
A)AB
B)BC
C)CD
D)DE

A)AB
B)BC
C)CD
D)DE

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
Which phases or states are present within the CD segment in the figure shown below? 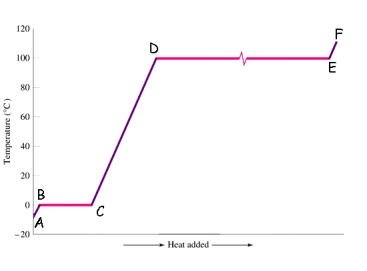
A)solid + liquid
B)solid only
C)liquid only
D)liquid + gas

A)solid + liquid
B)solid only
C)liquid only
D)liquid + gas

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
Which phase change represents melting?
A)Liquid to solid
B)Solid to liquid
C)Liquid to gas
D)Gas to liquid
A)Liquid to solid
B)Solid to liquid
C)Liquid to gas
D)Gas to liquid

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following reactions would produce water?
A)Fe(s)+ HNO3(aq)
B)NaCl(aq)+ KOH(aq)
C)C3H8(g)+ O2(g)
D)H2(g)+ Zn(s)
A)Fe(s)+ HNO3(aq)

B)NaCl(aq)+ KOH(aq)

C)C3H8(g)+ O2(g)
D)H2(g)+ Zn(s)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
For any substance,which phase of matter contains the greatest amount of energy?
A)Solid
B)Liquid
C)Gas
A)Solid
B)Liquid
C)Gas

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
The amount of energy required to change one gram of a liquid,at its boiling point,to a gas is called its heat of
A)fusion.
B)freezing.
C)vaporization.
D)sublimation.
A)fusion.
B)freezing.
C)vaporization.
D)sublimation.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
As the external pressure on a liquid increases,its boiling point temperature
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
The freezing point of a substance is the temperature at which its
A)solid phase is in equilibrium with its gaseous phase.
B)liquid phase is in equilibrium with its gaseous phase.
C)solid phase is in equilibrium with its liquid phase.
A)solid phase is in equilibrium with its gaseous phase.
B)liquid phase is in equilibrium with its gaseous phase.
C)solid phase is in equilibrium with its liquid phase.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
At which external pressure will water boil at the highest temperature?
A)0.5 atm
B)1.0 atm
C)1.5 atm
D)2.0 atm
A)0.5 atm
B)1.0 atm
C)1.5 atm
D)2.0 atm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
A sample of water at 20. ° C contains
A)covalent bonds only.
B)hydrogen bonds only.
C)both,covalent and hydrogen bonds.
D)neither,covalent nor hydrogen bonds.
A)covalent bonds only.
B)hydrogen bonds only.
C)both,covalent and hydrogen bonds.
D)neither,covalent nor hydrogen bonds.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Hydrogen bonds form between molecules of
A)H2O
B)H2S
C)H2Se
D)H2Te
A)H2O
B)H2S
C)H2Se
D)H2Te

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Approximately,what percentage of the Earth's surface is covered with water?
A)25 %
B)50 %
C)75 %
D)100 %
A)25 %
B)50 %
C)75 %
D)100 %

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
What is the normal freezing point of water?
A)0 K
B)32 K
C)273 K
D)373 K
A)0 K
B)32 K
C)273 K
D)373 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
When 5.00 g of Al2(SO4)3
 18H2O are heated until it dehydrates,how many grams of water are produced?
18H2O are heated until it dehydrates,how many grams of water are produced?
A)2.77 g
B)4.74 g
C)2.43 g
D)4.51 g
 18H2O are heated until it dehydrates,how many grams of water are produced?
18H2O are heated until it dehydrates,how many grams of water are produced?A)2.77 g
B)4.74 g
C)2.43 g
D)4.51 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
The amount of energy required to change one gram of a solid,at its freezing point,to a liquidis called its heat of
A)sublimation.
B)vaporization.
C)fusion.
D)condensation.
A)sublimation.
B)vaporization.
C)fusion.
D)condensation.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
Which substance contains molecules that will not form hydrogen bonds?
A)Hydrogen
B)Hydrogen fluoride
C)Water
D)Ammonia
A)Hydrogen
B)Hydrogen fluoride
C)Water
D)Ammonia

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Hydrogen bonds will form between molecules of compounds in which hydrogen is bonded to atoms of elements with
A)high electronegativity and large atomic radius.
B)high electronegativity and small atomic radius.
C)low electronegativity and large atomic radius.
D)low electronegativity and small atomic radius.
A)high electronegativity and large atomic radius.
B)high electronegativity and small atomic radius.
C)low electronegativity and large atomic radius.
D)low electronegativity and small atomic radius.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
The normal freezing point of water is zero degrees Celsius.What is its normal melting point?
A)-1° C
B)0° C
C)1° C
D)100° C
A)-1° C
B)0° C
C)1° C
D)100° C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
How many moles of water will be produced when 15.0 g of calcium chloride dihydrate are heated?
A)0.134 moles
B)0.0510 moles
C)0.204 moles
D)0.102 moles
A)0.134 moles
B)0.0510 moles
C)0.204 moles
D)0.102 moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
The normal boiling point of a liquid is the temperature at which its vapor pressure equals
A)100 torr
B)337 torr
C)373 torr
D)760 torr
A)100 torr
B)337 torr
C)373 torr
D)760 torr

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
Hydrogen bonding will not occur in molecules that contain bonds between hydrogen and
A)fluorine.
B)oxygen.
C)bromine.
D)nitrogen.
A)fluorine.
B)oxygen.
C)bromine.
D)nitrogen.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
Which phase change represents freezing?
A)Liquid to solid
B)Solid to liquid
C)Liquid to gas
D)Gas to liquid
A)Liquid to solid
B)Solid to liquid
C)Liquid to gas
D)Gas to liquid

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Which is a basic anhydride?
A)Carbon dioxide
B)Carbon monoxide
C)Sulfur dioxide
D)Calcium oxide
A)Carbon dioxide
B)Carbon monoxide
C)Sulfur dioxide
D)Calcium oxide

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
What type of bond exists between water molecules?
A)Polar covalent
B)Nonpolar covalent
C)Ionic
D)Hydrogen bond
A)Polar covalent
B)Nonpolar covalent
C)Ionic
D)Hydrogen bond

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
The high boiling point of water is due to
A)polar covalent bonds.
B)nonpolar covalent bonds.
C)ionic bonds.
D)hydrogen bonds.
A)polar covalent bonds.
B)nonpolar covalent bonds.
C)ionic bonds.
D)hydrogen bonds.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
Which is an acidic anhydride?
A)Strontium oxide
B)Potassium oxide
C)Barium oxide
D)Dinitrogen pentoxide
A)Strontium oxide
B)Potassium oxide
C)Barium oxide
D)Dinitrogen pentoxide

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
Which is an acidic anhydride?
A)Magnesium oxide
B)Sulfur trioxide
C)Lithium oxide
D)Sodium oxide
A)Magnesium oxide
B)Sulfur trioxide
C)Lithium oxide
D)Sodium oxide

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
What mass of water is found in one mole of magnesium sulfate heptahydrate?
A)120.37 g
B)138.39 g
C)126.14 g
D)246.51 g
A)120.37 g
B)138.39 g
C)126.14 g
D)246.51 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
How many molecules of water are contained in one formula unit of magnesium sulfate heptahydrate?
A)6
B)7
C)8
D)24
A)6
B)7
C)8
D)24

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
Which bond type is weakest?
A)Polar covalent bond
B)Nonpolar covalent bond
C)Ionic bond
D)Hydrogen bond
A)Polar covalent bond
B)Nonpolar covalent bond
C)Ionic bond
D)Hydrogen bond

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
The low equilibrium vapor pressure of water is due to
A)polar covalent bonds.
B)nonpolar covalent bonds.
C)ionic bonds.
D)hydrogen bonds.
A)polar covalent bonds.
B)nonpolar covalent bonds.
C)ionic bonds.
D)hydrogen bonds.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
Which is the anhydride of sulfuric acid?
A)H2SO3
B)H2SO4
C)SO2
D)SO3
A)H2SO3
B)H2SO4
C)SO2
D)SO3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
How many grams of water would react with 27.5 g of calcium metal?
A)27.5 g
B)12.4 g
C)6.18 g
D)24.7 g
A)27.5 g
B)12.4 g
C)6.18 g
D)24.7 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
Which is the anhydride of nitric acid?
A)NO3
B)NO2
C)N2O4
D)N2O5
A)NO3
B)NO2
C)N2O4
D)N2O5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Which is the anhydride of calcium hydroxide?
A)CaOH
B)Ca(OH)2
C)CaO
D)CaO2
A)CaOH
B)Ca(OH)2
C)CaO
D)CaO2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
Use the following information to select the substance with the lowest boiling point. 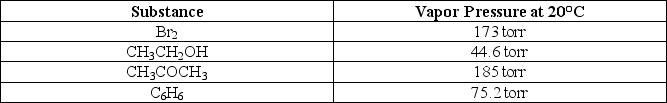
A)Br2
B)CH3CH2OH
C)CH3COCH3
D)C6H6

A)Br2
B)CH3CH2OH
C)CH3COCH3
D)C6H6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
Which is the anhydride of sodium hydroxide?
A)NaO
B)Na2O
C)NaOH
D)Na(OH)2
A)NaO
B)Na2O
C)NaOH
D)Na(OH)2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
What is the maximum density of water?
A)0.804 g/mL
B)1.00 g/mL
C)18.02 g/mL
D)22.4 g/mL
A)0.804 g/mL
B)1.00 g/mL
C)18.02 g/mL
D)22.4 g/mL

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
Which is a basic anhydride?
A)Sodium oxide
B)Nitrogen dioxide
C)Dinitrogen pentoxide
D)Silicon dioxide
A)Sodium oxide
B)Nitrogen dioxide
C)Dinitrogen pentoxide
D)Silicon dioxide

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Hard water contains salts of
A)potassium and sodium.
B)calcium and magnesium.
C)barium and lithium.
D)barium and sodium.
A)potassium and sodium.
B)calcium and magnesium.
C)barium and lithium.
D)barium and sodium.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
Which gas is produced when active metals react with cold water?
A)Oxygen
B)Carbon dioxide
C)Hydrogen
D)Nitrogen
A)Oxygen
B)Carbon dioxide
C)Hydrogen
D)Nitrogen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
What type of bond exists within the water molecule?
A)Polar covalent
B)Nonpolar covalent
C)Ionic
D)Hydrogen bond
A)Polar covalent
B)Nonpolar covalent
C)Ionic
D)Hydrogen bond

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
A sample of liquid water at 100. °C absorbs 113 kJ of heat energy.How much of the water will be converted to steam? The heat of vaporization of water is 2.26 kJ/g.
A)50.0 g
B)0.0200 g
C)255 g
D)113 g
A)50.0 g
B)0.0200 g
C)255 g
D)113 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following compounds will not exhibit hydrogen bonding?
A)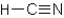
B)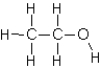
C)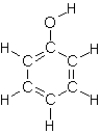
D)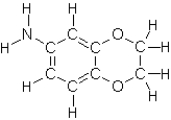
A)
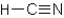
B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
What quantity of heat must be removed from 20.0 g of liquid water at 0 °C to completely freeze the water? The heat of fusion of ice is 335 J/g.
A)315 J
B)16.8 J
C)6700 J
D)0.0597 J
A)315 J
B)16.8 J
C)6700 J
D)0.0597 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following substances would exhibit hydrogen bonding?
A)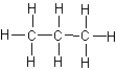
B)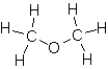
C)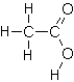
D)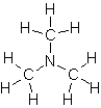
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
The term that best describes the rise of water up a thin glass cylinder is
A)vapor pressure.
B)capillary action.
C)cohesive force.
D)adhesive force.
A)vapor pressure.
B)capillary action.
C)cohesive force.
D)adhesive force.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
The chemical formula for calcium chlorate dihydrate is:
A)CaCl2
 2H2O
2H2O
B)Ca(ClO2)2
 2H2O
2H2O
C)Ca(ClO3)2
 2H2O
2H2O
D)Ca(ClO3)2H2
A)CaCl2
 2H2O
2H2OB)Ca(ClO2)2
 2H2O
2H2OC)Ca(ClO3)2
 2H2O
2H2OD)Ca(ClO3)2H2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
The name for (NH4)3PO4
 3H2O is:
3H2O is:
A)ammonium phosphate trihydrate
B)triammonia phosphate triwater
C)triammonium phosphate trihydrate
D)ammonium phosphide trihydrate
 3H2O is:
3H2O is:A)ammonium phosphate trihydrate
B)triammonia phosphate triwater
C)triammonium phosphate trihydrate
D)ammonium phosphide trihydrate

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
What amount of heat is required to convert 3.00 kg of water at its boiling point to steam? The heat of vaporization of water is 2.26 kJ/g.
A)6.78 kJ
B)6780 kJ
C)1330 kJ
D)1.33 kJ
A)6.78 kJ
B)6780 kJ
C)1330 kJ
D)1.33 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
What is the percent water in MgSO4.7H2O?
A)2.05 %
B)51.2 %
C)0.488 %
D)48.8 %
A)2.05 %
B)51.2 %
C)0.488 %
D)48.8 %

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
What is the percent water in copper(II)sulfate pentahydrate?
A)22.3 %
B)36.1 %
C)63.9 %
D)77.7 %
A)22.3 %
B)36.1 %
C)63.9 %
D)77.7 %

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
A sample of water at 10.0 ° C absorbs 4410 J of heat energy.The temperature of the sample increases to 72.0 °C.What is the mass of the water? The specific heat of liquid water is 4.184 J/g°C.
A)14.7 g
B)17.0 g
C)256 g
D)297 g
A)14.7 g
B)17.0 g
C)256 g
D)297 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following properties of water is not affected by hydrogen bonding?
A)Boiling point
B)Freezing point
C)Vapor pressure
D)Molar mass
A)Boiling point
B)Freezing point
C)Vapor pressure
D)Molar mass

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
An 18.0 g sample of liquid water at 42.0 ° C releases 979 J of heat energy.What is the final temperature of the water? The specific heat of liquid water is 4.184 J/g °C.
A)13.0 ° C
B)29.0 ° C
C)36.0 ° C
D)55.0 ° C
A)13.0 ° C
B)29.0 ° C
C)36.0 ° C
D)55.0 ° C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
A 32.0 g sample of liquid water at 21.0 ° C absorbs 2276 J of heat energy.What will be the final temperature of the water? The specific heat of liquid water is 4.185 J/g °C.
A)4.0 0 C
B)17.0 0 C
C)38.0 0 C
D)58.0 0 C
A)4.0 0 C
B)17.0 0 C
C)38.0 0 C
D)58.0 0 C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Hydrogen bonding
A)occurs only between water molecules.
B)is stronger than covalent bonding.
C)can occur between ammonia and water.
D)results from strong attractive forces in ionic compounds.
A)occurs only between water molecules.
B)is stronger than covalent bonding.
C)can occur between ammonia and water.
D)results from strong attractive forces in ionic compounds.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
A hydrogen bond is
A)a covalent bond between water molecules
B)a dipole-dipole attraction between molecules that contain H bonded to F,O,or N.
C)an ionic bond between water molecules.
D)a covalent bond between molecules that contain H bonded to F,O,or N.
A)a covalent bond between water molecules
B)a dipole-dipole attraction between molecules that contain H bonded to F,O,or N.
C)an ionic bond between water molecules.
D)a covalent bond between molecules that contain H bonded to F,O,or N.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
What quantity of heat is required to change 40.0 g of ice at melting point to liquid water? The heat of fusion of ice is 335 J/g.
A)13400 J
B)0.119 J
C)8.38 J
D)375 J
A)13400 J
B)0.119 J
C)8.38 J
D)375 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
What quantity of heat is required to convert 40.0 g of liquid water at its boiling point to steam? The heat of vaporization of water is 2.26 kJ/g.
A)17.7 kJ
B)0.0565 kJ
C)42.3 kJ
D)90.4 kJ
A)17.7 kJ
B)0.0565 kJ
C)42.3 kJ
D)90.4 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
What quantity of heat is required to change the temperature of 30.0 g of water at 10.0 °C to 25.0 °C? The specific heat of liquid water is 4.184 J/g °C.
A)62.8 J
B)126 J
C)107 J
D)1880 J
A)62.8 J
B)126 J
C)107 J
D)1880 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
A sample of ice at 0° C absorbs 6030 J of heat energy.How much of the ice can melt? The heat of fusion of ice is 335 J/g.
A)6030 g
B)2.02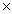 10 6 g
10 6 g
C)18.0 g
D)5700 g
A)6030 g
B)2.02
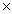 10 6 g
10 6 gC)18.0 g
D)5700 g

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


