Deck 17: Oxidationreduction
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 17: Oxidationreduction
1
In an electrolytic cell
A)a chemical reaction occurs spontaneously and releases energy as electricity.
B)electricity causes a chemical reaction to occur.
C)a physical change occurs spontaneously and releases energy as electricity.
D)electricity causes a physical change to occur.
A)a chemical reaction occurs spontaneously and releases energy as electricity.
B)electricity causes a chemical reaction to occur.
C)a physical change occurs spontaneously and releases energy as electricity.
D)electricity causes a physical change to occur.
electricity causes a chemical reaction to occur.
2
Which species is reduced in the following equation? 2 Ca + O2 2 CaO
A)Ca
B)O2
C)Ca+2
D)O-2
A)Ca
B)O2
C)Ca+2
D)O-2
O2
3
During a redox reaction,the oxidation number of the species reduced will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.
decrease.
4
The reducing agent is
A)the species that is reduced.
B)the species with a negative charge.
C)the species that loses electrons.
D)the species that gains electrons.
A)the species that is reduced.
B)the species with a negative charge.
C)the species that loses electrons.
D)the species that gains electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
What is the oxidation number of oxygen in OF2?
A)0
B)+2
C)-1
D)-2
A)0
B)+2
C)-1
D)-2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
What always occurs at the cathode in an electrolytic cell?
A)Protons are gained
B)Protons are lost
C)Electrons are gained
D)Electrons are lost
A)Protons are gained
B)Protons are lost
C)Electrons are gained
D)Electrons are lost

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
What is the oxidation number of manganese in MnO2?
A)+2
B)-4
C)+4
D)0
A)+2
B)-4
C)+4
D)0

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
Reduction is the
A)gain of protons.
B)loss of protons.
C)gain of electrons.
D)loss of electrons.
A)gain of protons.
B)loss of protons.
C)gain of electrons.
D)loss of electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Which species is oxidized in the following equation? 2 Na + Cl2 2 NaCl
A)Na
B)Cl2
C)Na+1
D)Cl-1
A)Na
B)Cl2
C)Na+1
D)Cl-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
In a voltaic cell
A)a chemical reaction occurs spontaneously and releases energy as electricity.
B)electricity causes a chemical reaction to occur.
C)a physical change occurs spontaneously and releases energy as electricity.
D)electricity causes a physical change to occur.
A)a chemical reaction occurs spontaneously and releases energy as electricity.
B)electricity causes a chemical reaction to occur.
C)a physical change occurs spontaneously and releases energy as electricity.
D)electricity causes a physical change to occur.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
What always travels through the external circuit (wire)in a voltaic cell?
A)Protons
B)Electrons
C)Neutrons
D)Ions
A)Protons
B)Electrons
C)Neutrons
D)Ions

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
What is the oxidation number of bromine in Br2?
A)0
B)-1
C)+7
D)-5
A)0
B)-1
C)+7
D)-5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
The oxidizing agent is
A)the species that is oxidized.
B)the species with a positive charge.
C)the species that loses electrons.
D)the species that gains electrons.
A)the species that is oxidized.
B)the species with a positive charge.
C)the species that loses electrons.
D)the species that gains electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
What always occurs at the anode in an electrolytic cell?
A)Protons are gained
B)Protons are lost
C)Electrons are gained
D)Electrons are lost
A)Protons are gained
B)Protons are lost
C)Electrons are gained
D)Electrons are lost

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
What always travels through the salt bridge in a voltaic cell?
A)Protons
B)Electrons
C)Neutrons
D)Ions
A)Protons
B)Electrons
C)Neutrons
D)Ions

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
During a redox reaction,the oxidation number of the species oxidized will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
What is the oxidation number of chromium in Cr2O7-2?
A)+12
B)0
C)+6
D)-4
A)+12
B)0
C)+6
D)-4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Oxidation is the
A)gain of protons.
B)loss of protons.
C)gain of electrons.
D)loss of electrons.
A)gain of protons.
B)loss of protons.
C)gain of electrons.
D)loss of electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
What is the oxidation number of chlorine in HClO4?
A)0
B)+7
C)-1
D)-3
A)0
B)+7
C)-1
D)-3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
What is the oxidation number of oxygen in H2O2?
A)-2
B)0
C)+2
D)-1
A)-2
B)0
C)+2
D)-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
How many moles of electrons are gained when four moles of fluorine atoms are reduced tofluoride ions?
A)1
B)2
C)4
D)8
A)1
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Which occurs when a calcium atom is oxidized?
A)Two protons are lost
B)Two protons are gained
C)Two electrons are lost
D)Two electrons are gained
A)Two protons are lost
B)Two protons are gained
C)Two electrons are lost
D)Two electrons are gained

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
In this chemical process,the oxidation numbers of aluminum on the reactant and the product are: 2 Al2O3 4 Al + 3 O2
A)+2,+3
B)+3,0
C)+2,+4
D)0,+4
A)+2,+3
B)+3,0
C)+2,+4
D)0,+4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
How many moles of electrons are lost when two moles of aluminum atoms are oxidized toaluminum ions?
A)2
B)3
C)6
D)9
A)2
B)3
C)6
D)9

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
Which species is oxidized in the following equation? 2 H2O 2 H2 + O2
A)H2
B)O2
C)H+1
D)O-2
A)H2
B)O2
C)H+1
D)O-2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
Which species is the oxidizing agent in the following equation?
2 H2O 2 H2 + O2
A)H+1
B)O-2
C)H2
D)O2
2 H2O 2 H2 + O2
A)H+1
B)O-2
C)H2
D)O2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of electron are lost when one mole of barium atoms is oxidized to barium ions?
A)2
B)1
C)4
D)3
A)2
B)1
C)4
D)3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Which is the correct reduction half-reaction for the following equation?
Zn + 2 HCl ZnCl2 + H2
A)Zn Zn+2 + 2 e-1
B)Zn+2 + 2 e-1 Zn
C)2 H+1 + 2 e-1 H2
D)H2 2 H+1 + 2 e-1
Zn + 2 HCl ZnCl2 + H2
A)Zn Zn+2 + 2 e-1
B)Zn+2 + 2 e-1 Zn
C)2 H+1 + 2 e-1 H2
D)H2 2 H+1 + 2 e-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Which is the correct reduction half-reaction for the following equation?
Ba + CuO BaO + Cu
A)Ba+2 + 2 e-1 Ba
B)Cu Cu+2 + 2 e-1
C)Ba Ba+2 + 2 e-1
D)Cu+2 + 2 e-1 Cu
Ba + CuO BaO + Cu
A)Ba+2 + 2 e-1 Ba
B)Cu Cu+2 + 2 e-1
C)Ba Ba+2 + 2 e-1
D)Cu+2 + 2 e-1 Cu

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Which species is the reducing agent in the following equation?
2 Al2O3 4 Al + 3O2
A)Al+3
B)O-2
C)Al
D)O2
2 Al2O3 4 Al + 3O2
A)Al+3
B)O-2
C)Al
D)O2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Which is the correct oxidation half-reaction for the following equation?
Zn + 2 Ag+1 Zn+2 + 2 Ag
A)Ag Ag+1 + 1 e-1
B)Ag+1 + 1 e-1 Ag
C)Zn+2 + 2 e-1 Zn
D)Zn Zn+2 + 2 e-1
Zn + 2 Ag+1 Zn+2 + 2 Ag
A)Ag Ag+1 + 1 e-1
B)Ag+1 + 1 e-1 Ag
C)Zn+2 + 2 e-1 Zn
D)Zn Zn+2 + 2 e-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Which species is the reducing agent in the following equation?
2 Ca + O2 2 CaO
A)Ca
B)O2
C)Ca+2
D)O-2
2 Ca + O2 2 CaO
A)Ca
B)O2
C)Ca+2
D)O-2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
In a redox reaction,the total number of electrons gained is
A)greater than the total number of electrons lost.
B)less than the total number of electrons lost.
C)equal to the number of electrons lost.
D)not related at all to the number of electrons lost.
A)greater than the total number of electrons lost.
B)less than the total number of electrons lost.
C)equal to the number of electrons lost.
D)not related at all to the number of electrons lost.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
How many moles of electrons are gained when three moles of oxygen atoms are reduced tooxide ions?
A)2
B)3
C)4
D)6
A)2
B)3
C)4
D)6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Which occurs when an oxygen atom is reduced?
A)Two protons are gained
B)Two protons are lost
C)Two electrons are gained
D)Two electrons are lost
A)Two protons are gained
B)Two protons are lost
C)Two electrons are gained
D)Two electrons are lost

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
A redox reaction is a reaction in which
A)only oxidation occurs.
B)only reduction occurs.
C)both reduction and oxidation occur.
D)neither oxidation nor reduction occur.
A)only oxidation occurs.
B)only reduction occurs.
C)both reduction and oxidation occur.
D)neither oxidation nor reduction occur.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Which occurs when a sodium atom is oxidized?
A)One electron is gained
B)One electron is lost
C)One proton is gained
D)One proton is lost
A)One electron is gained
B)One electron is lost
C)One proton is gained
D)One proton is lost

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Which occurs when a chlorine atom is reduced?
A)One electron is gained
B)One electron is lost
C)One proton is gained
D)One proton is lost
A)One electron is gained
B)One electron is lost
C)One proton is gained
D)One proton is lost

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
Which is the correct oxidation half-reaction for the following equation?
2 Na + 2 H2O 2 NaOH + H2
A)Na+1 + e-1 Na
B)Na Na+1 + e-1
C)2 H+1 + 2 e-1 H2
D)H2 2 H+1 + 2 e-1
2 Na + 2 H2O 2 NaOH + H2
A)Na+1 + e-1 Na
B)Na Na+1 + e-1
C)2 H+1 + 2 e-1 H2
D)H2 2 H+1 + 2 e-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
Which species is the oxidizing agent in the following equation?
2 Na + Cl2 2 NaCl
A)Na
B)Cl2
C)Na+1
D)Cl-1
2 Na + Cl2 2 NaCl
A)Na
B)Cl2
C)Na+1
D)Cl-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
What is the oxidation number of carbon in CO2?
A)-4
B)-2
C)+2
D)+4
A)-4
B)-2
C)+2
D)+4

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Which element forms ions that will migrate toward the cathode in an electrolytic cell?
A)Bromine
B)Oxygen
C)Iodine
D)Silver
A)Bromine
B)Oxygen
C)Iodine
D)Silver

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
What is the oxidation number of oxygen in MgCrO4?
A)-2
B)0
C)+2
D)-1
A)-2
B)0
C)+2
D)-1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
Which element forms ions that will migrate toward the anode in an electrolytic cell?
A)Silver
B)Chlorine
C)Copper
D)Sodium
A)Silver
B)Chlorine
C)Copper
D)Sodium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
What is the oxidation number of sulfur in H2SO4?
A)+2
B)+4
C)+6
D)-2
A)+2
B)+4
C)+6
D)-2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Which species is oxidized in the following equation?
2 Na + 2 H+1 2 Na+1 + H2
A)Na+1
B)H2
C)Na
D)H+1
2 Na + 2 H+1 2 Na+1 + H2
A)Na+1
B)H2
C)Na
D)H+1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
Which element forms ions that will migrate toward the cathode in an electrolytic cell?
A)Copper
B)Chlorine
C)Oxygen
D)Fluorine
A)Copper
B)Chlorine
C)Oxygen
D)Fluorine

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
What is the oxidation number of hydrogen in NH3?
A)-3
B)-1
C)+1
D)+3
A)-3
B)-1
C)+1
D)+3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
What is the oxidation number of iron in Fe2O3?
A)+2
B)+4
C)+3
D)+6
A)+2
B)+4
C)+3
D)+6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
In the following reaction,the copper(II)ions Ni + Cu+2 Ni+2 + Cu
A)gain electrons.
B)lose electrons.
C)gain protons.
D)lose protons.
A)gain electrons.
B)lose electrons.
C)gain protons.
D)lose protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
Which element forms ions that will migrate toward the anode in an electrolytic cell?
A)Copper
B)Potassium
C)Bromine
D)Gold
A)Copper
B)Potassium
C)Bromine
D)Gold

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
In the following reaction,the chlorine atoms 2 Na + Cl2 2 NaCl
A)gain electrons.
B)lose electrons.
C)gain protons.
D)lose protons.
A)gain electrons.
B)lose electrons.
C)gain protons.
D)lose protons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
In the following redox reaction F2(g)+ 2NaI(aq)  I2(s)+ 2NaF(aq)
I2(s)+ 2NaF(aq)
The total number of moles of electrons transferred between one mole of the oxidizing and reducing agents is
A)one mole
B)three moles
C)0.5 mole
D)two moles
 I2(s)+ 2NaF(aq)
I2(s)+ 2NaF(aq)The total number of moles of electrons transferred between one mole of the oxidizing and reducing agents is
A)one mole
B)three moles
C)0.5 mole
D)two moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
When the following reaction is balanced in basic media KOH + Cl2  KCl + KClO
KCl + KClO
Moles of water molecules will appear on the side of the balanced equation.
A)two,reactant
B)four,product
C)two,product
D)four,reactant
 KCl + KClO
KCl + KClOMoles of water molecules will appear on the side of the balanced equation.
A)two,reactant
B)four,product
C)two,product
D)four,reactant

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
Which is conserved in all oxidation-reduction reactions?
A)Mass,only
B)Charge,only
C)Both mass and charge
D)Neither mass nor charge
A)Mass,only
B)Charge,only
C)Both mass and charge
D)Neither mass nor charge

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
What is the oxidation number of hydrogen in H2O2?
A)-2
B)-1
C)+1
D)+2
A)-2
B)-1
C)+1
D)+2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
In the following reaction,the zinc atoms Zn + 2 Ag+1 Zn+2 + 2 Ag
A)gain protons.
B)lose protons.
C)gain electrons.
D)lose electrons.
A)gain protons.
B)lose protons.
C)gain electrons.
D)lose electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
In the following reaction,the hydrogen atoms 2 H2 + O2 2 H2O
A)gain protons.
B)lose protons.
C)gain electrons.
D)lose electrons.
A)gain protons.
B)lose protons.
C)gain electrons.
D)lose electrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
What is the oxidation number of oxygen in O2?
A)-2
B)-1
C)0
D)+2
A)-2
B)-1
C)0
D)+2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
In the following redox reaction Fe2O3 + C  2Fe + CO2
2Fe + CO2
The total number of moles of electrons transferred between one mole of the oxidizing and reducing agents is
A)two moles
B)one mole
C)0.5 mole
D)three moles
 2Fe + CO2
2Fe + CO2The total number of moles of electrons transferred between one mole of the oxidizing and reducing agents is
A)two moles
B)one mole
C)0.5 mole
D)three moles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
When the following reaction is balanced in acidic media Cu + 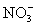
 Cu2+ + NO
Cu2+ + NO
One of the terms in the balanced equation will be:
A)3H2O
B)8H+
C)2Cu2+
D)4H+
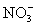
 Cu2+ + NO
Cu2+ + NOOne of the terms in the balanced equation will be:
A)3H2O
B)8H+
C)2Cu2+
D)4H+

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following species will displace nickel from Ni(C2H3O2)2(aq):
A)Cu(s)
B)Hg(l)
C)Ag(s)
D)Na(s)
A)Cu(s)
B)Hg(l)
C)Ag(s)
D)Na(s)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
In a voltaic cell,oxidation occurs at the
A)Anode
B)Cathode
C)Internal circuit
D)External circuit
A)Anode
B)Cathode
C)Internal circuit
D)External circuit

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
The products of the reaction between solid magnesium metal and water are
A)Mg(OH)2(aq)and H2(g)
B)MgO2(aq)and H2(g)
C)MgH2(aq)+ O2(g)
D)No reaction takes place.
A)Mg(OH)2(aq)and H2(g)
B)MgO2(aq)and H2(g)
C)MgH2(aq)+ O2(g)
D)No reaction takes place.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
All the following are true for a galvanic cell except
A)Oxidation occurs at the cathode
B)A spontaneous reaction takes place in the cell
C)Cations are attracted to the cathode
D)The salt bridge allows the migration of ions
A)Oxidation occurs at the cathode
B)A spontaneous reaction takes place in the cell
C)Cations are attracted to the cathode
D)The salt bridge allows the migration of ions

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
In the electrolysis of fused (molten)sodium chloride,the product at the anode is
A)Na
B)Na+1
C)Cl-1
D)Cl2
A)Na
B)Na+1
C)Cl-1
D)Cl2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following species will displace lead from Pb(NO3)2(aq):
A)Au
B)Hg
C)Cr3+
D)Ba
A)Au
B)Hg
C)Cr3+
D)Ba

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
In the electrolysis of fused (molten)sodium chloride,the product at the cathode is
A)Na+1
B)Na
C)Cl-1
D)Cl2
A)Na+1
B)Na
C)Cl-1
D)Cl2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
When the following equation is balanced in acidic media 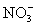 + S
+ S  NO2 +
NO2 + 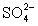 one of the terms in the balanced equation will be:
one of the terms in the balanced equation will be:
A)8H+
B)2H2O
C)6H2O
D)2NO2
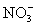 + S
+ S  NO2 +
NO2 + 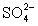 one of the terms in the balanced equation will be:
one of the terms in the balanced equation will be:A)8H+
B)2H2O
C)6H2O
D)2NO2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
Nitrogen has a +3 oxidation state in
A)N2O
B)N2O3
C)N2O4
D)NO
A)N2O
B)N2O3
C)N2O4
D)NO

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
In MnCr2O7,the oxidation number of chromium is
A)+2
B)-2
C)+12
D)+6
A)+2
B)-2
C)+12
D)+6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
Which does not involve oxidation-reduction?
A)Burning sodium in chlorine
B)The chemical formation of iron(II)sulfide from its elements
C)Decomposition of potassium chlorate
D)Neutralization of sodium hydroxide by sulfuric acid
A)Burning sodium in chlorine
B)The chemical formation of iron(II)sulfide from its elements
C)Decomposition of potassium chlorate
D)Neutralization of sodium hydroxide by sulfuric acid

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
In the electrolysis of fused (molten)calcium chloride,the product at the cathode is
A)Ca+2
B)Cl-1
C)Cl2
D)Ca
A)Ca+2
B)Cl-1
C)Cl2
D)Ca

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
Moving from the top to the bottom of the activity series of the metals
A)arranges the metals by increasing amount of electrons lost.
B)arranges the metals by decreasing ease of reduction.
C)arranges the metals by decreasing ease of oxidation.
D)arranges the metals by increasing reactivity.
A)arranges the metals by increasing amount of electrons lost.
B)arranges the metals by decreasing ease of reduction.
C)arranges the metals by decreasing ease of oxidation.
D)arranges the metals by increasing reactivity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
In an electrolytic cell the charge of the cathode is
A)Positive
B)Negative
C)Neutral
A)Positive
B)Negative
C)Neutral

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
In Ba(NO3)2,the oxidation number of nitrogen is
A)-3
B)-1
C)+4
D)+5
A)-3
B)-1
C)+4
D)+5

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Which is the principal characteristic of all redox reactions?
A)The formation of water
B)The formation of a gas or precipitate
C)An irreversible reaction
D)The transfer of electrons
A)The formation of water
B)The formation of a gas or precipitate
C)An irreversible reaction
D)The transfer of electrons

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
In a voltaic cell,reduction occurs at the
A)External circuit
B)Internal circuit
C)Cathode
D)Anode
A)External circuit
B)Internal circuit
C)Cathode
D)Anode

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
In the electrolysis of fused (molten)calcium chloride,the product at the anode is
A)Cl-1
B)Cl2
C)Ca+2
D)Ca
A)Cl-1
B)Cl2
C)Ca+2
D)Ca

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
The products of the reaction between iron metal and calcium chloride are
A)FeCl2(aq)+ Ca(s)
B)No reaction takes place.
C)FeCl(aq)+ CaCl(aq)
D)CaFe(s)+ Cl2(g)
A)FeCl2(aq)+ Ca(s)
B)No reaction takes place.
C)FeCl(aq)+ CaCl(aq)
D)CaFe(s)+ Cl2(g)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


