Deck 19: Introduction to Organic Chemistry Online Only
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 19: Introduction to Organic Chemistry Online Only
1
In which hydrocarbon series is there a single covalent bond between all carbon atoms?
A)Aromatics
B)Alkanes
C)Alkenes
D)Alkynes
A)Aromatics
B)Alkanes
C)Alkenes
D)Alkynes
Alkanes
2
Which compound is saturated?
A)CH4
B)C3H6
C)C2H2
D)C6H6
A)CH4
B)C3H6
C)C2H2
D)C6H6
CH4
3
CnH2n-6 is the general formula for the
A)Alkenes
B)Alkanes
C)Alkynes
D)Aromatics
A)Alkenes
B)Alkanes
C)Alkynes
D)Aromatics
Aromatics
4
How many pairs of electrons are shared in a single covalent bond?
A)One
B)Two
C)Three
D)Four
A)One
B)Two
C)Three
D)Four

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Which compound is more soluble in water?
A)ethane
B)ethene
C)dimethyl ether
D)ethanol
A)ethane
B)ethene
C)dimethyl ether
D)ethanol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which is saturated?
A)C2H4
B)C3H6
C)C6H8
D)C2H6
A)C2H4
B)C3H6
C)C6H8
D)C2H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Which hydrocarbon series contains a double covalent bond between carbon atoms?
A)Alkynes
B)Alkenes
C)Alkanes
D)Aromatics
A)Alkynes
B)Alkenes
C)Alkanes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Which hydrocarbon series contains a triple covalent bond between carbon atoms?
A)Alkynes
B)Alkanes
C)Alkenes
D)Aromatics
A)Alkynes
B)Alkanes
C)Alkenes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
How many pairs of electrons are shared in a triple covalent bond?
A)One
B)Two
C)Three
D)Four
A)One
B)Two
C)Three
D)Four

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
What type of compound is composed of only carbon and hydrogen atoms?
A)Carbohydrate
B)Hydrocarbon
C)Ester
D)Carboxylic acid
A)Carbohydrate
B)Hydrocarbon
C)Ester
D)Carboxylic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Which is unsaturated?
A)C2H6
B)C4H10
C)C5H12
D)C6H12
A)C2H6
B)C4H10
C)C5H12
D)C6H12

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following structures represents a molecule of benzene?
A)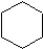
B)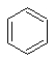
C)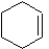
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
CnH2n is the general formula for the
A)Alkynes
B)Alkenes
C)Alkanes
D)Aromatics
A)Alkynes
B)Alkenes
C)Alkanes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Which hydrocarbon series is saturated?
A)Alkenes
B)Alkynes
C)Alkanes
D)Aromatics
A)Alkenes
B)Alkynes
C)Alkanes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
Alkyl groups have the general formula
A)CnH2n
B)CnH2n+1
C)CnH2n+2
D)CnHn+2
A)CnH2n
B)CnH2n+1
C)CnH2n+2
D)CnHn+2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Which hydrocarbon series contains a benzene ring?
A)Alkanes
B)Alkenes
C)Alkynes
D)Aromatics
A)Alkanes
B)Alkenes
C)Alkynes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
How many pairs of electrons are shared in a double covalent bond?
A)One
B)Two
C)Three
D)Four
A)One
B)Two
C)Three
D)Four

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
CnH2n+2 is the general formula for the
A)Alkenes
B)Alkynes
C)Alkanes
D)Aromatics
A)Alkenes
B)Alkynes
C)Alkanes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Which is unsaturated?
A)C3H8
B)C4H10
C)CH4
D)C5H8
A)C3H8
B)C4H10
C)CH4
D)C5H8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
CnH2n-2 is the general formula for the
A)Alkynes
B)Alkanes
C)Alkenes
D)Aromatics
A)Alkynes
B)Alkanes
C)Alkenes
D)Aromatics

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
Which has the greatest number of isomers?
A)CH4
B)C2H6
C)C3H8
D)C4H10
A)CH4
B)C2H6
C)C3H8
D)C4H10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
CH3CH=CHCH2CH3 is
A)pentane.
B)pentyne.
C)2-pentene.
D)3-pentene.
A)pentane.
B)pentyne.
C)2-pentene.
D)3-pentene.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
CH3CH2CH2CH(CH3)2 is
A)heptane.
B)2-methylpentane.
C)1,1-dimethylbutane.
D)hexane.
A)heptane.
B)2-methylpentane.
C)1,1-dimethylbutane.
D)hexane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
In any hydrocarbon series,as the number of carbon atoms increases,the number of isomers
A)increases.
B)decreases.
C)remains the same.
A)increases.
B)decreases.
C)remains the same.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Which has the greatest number of isomers?
A)C3H8
B)C12H26
C)C5H12
D)C7H16
A)C3H8
B)C12H26
C)C5H12
D)C7H16

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
C2H6 is
A)propane.
B)ethane.
C)butane.
D)methane.
A)propane.
B)ethane.
C)butane.
D)methane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
CH3CH2CH(CH3)2 is
A)1,1-dimethylpropane
B)3,3-dimethylpropane
C)2-methylbutane
D)4-methylbutane
A)1,1-dimethylpropane
B)3,3-dimethylpropane
C)2-methylbutane
D)4-methylbutane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which hydrocarbon can undergo an addition reaction?
A)CH4
B)C2H4
C)C2H6
D)C3H8
A)CH4
B)C2H4
C)C2H6
D)C3H8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
C3H8 is
A)octane.
B)butane.
C)propane.
D)methane.
A)octane.
B)butane.
C)propane.
D)methane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
Two or more different compounds with the same molecular formula are
A)Isomers
B)Isotopes
C)Hypermeres
D)Hypertopes
A)Isomers
B)Isotopes
C)Hypermeres
D)Hypertopes

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Which hydrocarbon can undergo an addition reaction?
A)C4H8
B)C3H8
C)C2H6
D)C6H6
A)C4H8
B)C3H8
C)C2H6
D)C6H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
CH3CH=CH2 is
A)propane.
B)1-methylethene.
C)propene.
D)butane.
A)propane.
B)1-methylethene.
C)propene.
D)butane.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
Which is benzene?
A)C3H3
B)C6H6
C)C8H8
D)C9H9
A)C3H3
B)C6H6
C)C8H8
D)C9H9

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Which is toluene?
A)C6H6
B)C7H8
C)C6H8
D)C8H7
A)C6H6
B)C7H8
C)C6H8
D)C8H7

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
The difference between any two consecutive members of a homologous series of hydrocarbons is
A)One carbon atom and one oxygen atom
B)One carbon atom and two hydrogen atoms
C)Two carbon atoms and one hydrogen atom
D)Two carbon atoms and two hydrogen atoms
A)One carbon atom and one oxygen atom
B)One carbon atom and two hydrogen atoms
C)Two carbon atoms and one hydrogen atom
D)Two carbon atoms and two hydrogen atoms

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
C2H2 is
A)methane.
B)methyne.
C)ethane.
D)ethyne.
A)methane.
B)methyne.
C)ethane.
D)ethyne.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Complete combustion of an alkane produces
A)CO2,only
B)H2O,only
C)CO2 and H2O
D)CO and H2O
A)CO2,only
B)H2O,only
C)CO2 and H2O
D)CO and H2O

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
Which hydrocarbon can undergo a substitution reaction?
A)C3H4
B)C2H4
C)C3H6
D)CH4
A)C3H4
B)C2H4
C)C3H6
D)CH4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Which is a carboxylic acid?
A)CH3OH
B)HCOOH
C)CH3OCH3
D)CH4
A)CH3OH
B)HCOOH
C)CH3OCH3
D)CH4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Which hydrocarbon can undergo a substitution reaction?
A)C2H2
B)C6H6
C)C3H6
D)C4H6
A)C2H2
B)C6H6
C)C3H6
D)C4H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Which is a ketone?
A)CH3COCH3
B)CH3COOH
C)C2H5OH
D)CH3CHO
A)CH3COCH3
B)CH3COOH
C)C2H5OH
D)CH3CHO

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
Which is an ether?
A)HCOOH
B)CH3COCH3
C)CH3OCH3
D)CH3OH
A)HCOOH
B)CH3COCH3
C)CH3OCH3
D)CH3OH

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Which is a trihydroxy alcohol?
A)3-pentanol
B)1,2,3-propanetriol
C)3-propanol
D)1-propanol
A)3-pentanol
B)1,2,3-propanetriol
C)3-propanol
D)1-propanol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which molecule contains seven carbon atoms?
A)3-methylpentane
B)Hexane
C)2-methylpropane
D)2,3-dimethylpentane
A)3-methylpentane
B)Hexane
C)2-methylpropane
D)2,3-dimethylpentane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
Which is the second member of the alkene series?
A)C2H6
B)C2H4
C)C3H8
D)C3H6
A)C2H6
B)C2H4
C)C3H8
D)C3H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
A molecule of propane and a molecule of propene have the same
A)general formula.
B)molecular formula.
C)structural formula.
D)number of carbon atoms.
A)general formula.
B)molecular formula.
C)structural formula.
D)number of carbon atoms.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
What is the total number of carbon atoms in a molecule of 2-methyl-4-ethyloctane?
A)8
B)11
C)14
D)17
A)8
B)11
C)14
D)17

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Which is an ester?
A)CH3OH
B)CH3CH2COOCH3
C)HCOOH
D)HCHO
A)CH3OH
B)CH3CH2COOCH3
C)HCOOH
D)HCHO

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
Which compound will form an aqueous solution that will turn blue litmus paper red?
A)CH3OH
B)CH3COOH
C)CH3COOCH3
D)HCHO
A)CH3OH
B)CH3COOH
C)CH3COOCH3
D)HCHO

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Which pair contains two molecules from the same homologous series of hydrocarbons?
A)CH4 and C2H2
B)C2H6 and C5H12
C)C2H6 and C3H6
D)C2H2 and C2H6
A)CH4 and C2H2
B)C2H6 and C5H12
C)C2H6 and C3H6
D)C2H2 and C2H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Which is a dihydroxy alcohol?
A)2-pentanol
B)2,3-butanediol
C)Ethanol
D)1,2,3-propanetriol
A)2-pentanol
B)2,3-butanediol
C)Ethanol
D)1,2,3-propanetriol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Which is a secondary alcohol?
A)1,2-propanediol
B)3-pentanol
C)Ethanol
D)1-propanol
A)1,2-propanediol
B)3-pentanol
C)Ethanol
D)1-propanol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
Which pair contains two molecules from the same homologous series of hydrocarbons?
A)C6H6 and C7H8
B)C3H8 and C3H6
C)C6H6 and C2H6
D)C6H6 and C7H6
A)C6H6 and C7H8
B)C3H8 and C3H6
C)C6H6 and C2H6
D)C6H6 and C7H6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Which contains seven carbon atoms?
A)2,2-dimethylbutane
B)2,2-dimethylpropane
C)Benzene
D)Methylbenzene
A)2,2-dimethylbutane
B)2,2-dimethylpropane
C)Benzene
D)Methylbenzene

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
What is the total number of carbon atoms in a molecule of toluene?
A)1
B)6
C)7
D)12
A)1
B)6
C)7
D)12

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Which molecule contains five carbon atoms?
A)Propane
B)2-methylpropane
C)Butane
D)2-methylbutane
A)Propane
B)2-methylpropane
C)Butane
D)2-methylbutane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
Which two compounds are isomers?
A)Methane and methanol
B)Propane and butane
C)Propane and propene
D)2-methylpropane and butane
A)Methane and methanol
B)Propane and butane
C)Propane and propene
D)2-methylpropane and butane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
Which is a primary alcohol?
A)Glycerol
B)1,2-ethanediol
C)Ethanol
D)1,2,3-propanetriol
A)Glycerol
B)1,2-ethanediol
C)Ethanol
D)1,2,3-propanetriol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which is an aldehyde?
A)HCOOH
B)C2H5OH
C)CH3COCH3
D)HCHO
A)HCOOH
B)C2H5OH
C)CH3COCH3
D)HCHO

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which two compounds are isomers?
A)Ethanol and 1-propanol
B)1-propanol and 2-propanol
C)2-propanol and 2-propanal
D)Propane and propyne
A)Ethanol and 1-propanol
B)1-propanol and 2-propanol
C)2-propanol and 2-propanal
D)Propane and propyne

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is not the correct name for the alkane shown with it?
A)C2H6,Ethane
B)C5H12,Propane
C)C7H16,Heptane
D)C10H22,Decane
A)C2H6,Ethane
B)C5H12,Propane
C)C7H16,Heptane
D)C10H22,Decane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
What is the name of CH3-O-CH2CH3?
A)Propanone
B)Propanal
C)Propanol
D)Methyl ethyl ether
A)Propanone
B)Propanal
C)Propanol
D)Methyl ethyl ether

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
Which compound is not a member of the same homologous series as the others?
A)C6H14
B)C5H10
C)CH4
D)C3H8
A)C6H14
B)C5H10
C)CH4
D)C3H8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
What is the name of CH3(CH2)4COOH?
A)Hexanoic acid
B)Heptanoic acid
C)Benzoic acid
D)Propanoic acid
A)Hexanoic acid
B)Heptanoic acid
C)Benzoic acid
D)Propanoic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
What is the name for the following hydrocarbon? 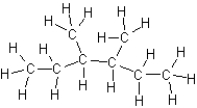
A)2-ethyl-3-methylpentane
B)3,4-dimethylhexane
C)2,3-diethylbutane
D)octane

A)2-ethyl-3-methylpentane
B)3,4-dimethylhexane
C)2,3-diethylbutane
D)octane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
What is the name of C6H5COOH?
A)Heptanoic acid
B)Hexanoic acid
C)Benzoic acid
D)Phenol
A)Heptanoic acid
B)Hexanoic acid
C)Benzoic acid
D)Phenol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
The number of possible isomers of C6H14 is
A)3
B)5
C)6
D)8
A)3
B)5
C)6
D)8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Which of these has no structural isomers?
A)C5H10
B)C4H10
C)C3H8
D)C6H12
A)C5H10
B)C4H10
C)C3H8
D)C6H12

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of CH3CH2COOCH3?
A)Methyl propanoate
B)Propyl methanoate
C)Methyl ethanoate
D)Methyl propyl ether
A)Methyl propanoate
B)Propyl methanoate
C)Methyl ethanoate
D)Methyl propyl ether

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction: CH2=CH2 + Br2 CH2BrCH2Br,is an example of
A)dehalogenation.
B)dehydration.
C)addition.
D)substitution.
A)dehalogenation.
B)dehydration.
C)addition.
D)substitution.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Name the following compound: 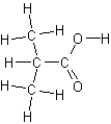
A)methyl butanoate
B)hydroxybutylketone
C)2-methylpropanal
D)2-methylpropanoic acid

A)methyl butanoate
B)hydroxybutylketone
C)2-methylpropanal
D)2-methylpropanoic acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Which is not an aromatic compound?
A)C6H6
B)C6H5OH
C)C6H14
D)C6H5CH3
A)C6H6
B)C6H5OH
C)C6H14
D)C6H5CH3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
The number of possible isomers of C5H12 is
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
The general formula for a ketone is
A)RCHO
B)ROR
C)RCOOR
D)R2CO
A)RCHO
B)ROR
C)RCOOR
D)R2CO

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
What is the maximum number of covalent bonds a carbon atom can form?
A)One
B)Two
C)Three
D)Four
A)One
B)Two
C)Three
D)Four

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is an isomer of butane?
A)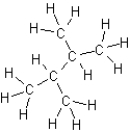
B)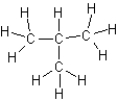
C)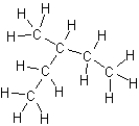
D)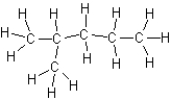
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds is a structural isomer of hexane?
A)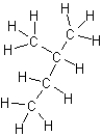
B)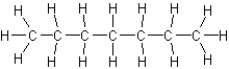
C)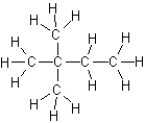
D)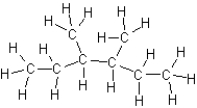
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
What is the name of CH3(CH2)2CH2Cl?
A)3-chlorobutane
B)1-chlorobutane
C)3-chloropropane
D)1-chloropropane
A)3-chlorobutane
B)1-chlorobutane
C)3-chloropropane
D)1-chloropropane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Para-dichlorobenzene is also known as
A)1,2-dichlorobenzene
B)1,3-dichlorobenzene
C)1,4-dichlorobenzene
D)1,5-dichlorobenzene
A)1,2-dichlorobenzene
B)1,3-dichlorobenzene
C)1,4-dichlorobenzene
D)1,5-dichlorobenzene

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Name the following compound: 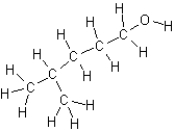
A)4-methyl-1-pentanol
B)2-methyl-5-pentanol
C)1-hexanol
D)4,4-dimethyl-1-butanol

A)4-methyl-1-pentanol
B)2-methyl-5-pentanol
C)1-hexanol
D)4,4-dimethyl-1-butanol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


