Deck 5: Early Atomic Theory and Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 5: Early Atomic Theory and Structure
1
What is the relative electrical charge of a proton?
A)-1
B)+1
C)-2
D)0
A)-1
B)+1
C)-2
D)0
+1
2
The Law of Multiple Proportions states that
A)atoms of one element may combine in different ratios to form more than one compound.
B)atoms of one element may combine in different ratios to form the same compound.
C)atoms of two or more elements may combine in different ratios to form more than one compound.
D)atoms of two or more elements may combine in different ratios to produce the same compound.
A)atoms of one element may combine in different ratios to form more than one compound.
B)atoms of one element may combine in different ratios to form the same compound.
C)atoms of two or more elements may combine in different ratios to form more than one compound.
D)atoms of two or more elements may combine in different ratios to produce the same compound.
atoms of two or more elements may combine in different ratios to form more than one compound.
3
The Law of Definite Composition states that
A)a compound always contains one element physically combined in variable proportions by mass.
B)a compound always contains one element chemically combined in a definite proportion by mass.
C)a compound always contains two or more elements physically combined in variable proportions by mass.
D)a compound always contains two or more elements chemically combined in a definite proportion by mass.
A)a compound always contains one element physically combined in variable proportions by mass.
B)a compound always contains one element chemically combined in a definite proportion by mass.
C)a compound always contains two or more elements physically combined in variable proportions by mass.
D)a compound always contains two or more elements chemically combined in a definite proportion by mass.
a compound always contains two or more elements chemically combined in a definite proportion by mass.
4
Which pair of formulas illustrates the Law of Multiple Proportions?
A)CH3Cl and CH3OH
B)H2O and HOH
C)CuCl2 and CuBr
D)H2O and H2O2
A)CH3Cl and CH3OH
B)H2O and HOH
C)CuCl2 and CuBr
D)H2O and H2O2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
As the distance between two particles with charges that repel each other increases,the force of repulsion will
A)increase
B)decrease
C)remain the same
A)increase
B)decrease
C)remain the same

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
How many types of electrical charge exist?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
Neutral atoms of a specific element may have different
A)atomic numbers.
B)number of protons.
C)number of electrons.
D)mass numbers.
A)atomic numbers.
B)number of protons.
C)number of electrons.
D)mass numbers.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
What is the relative electrical charge of a neutron?
A)-1
B)+1
C)+2
D)0
A)-1
B)+1
C)+2
D)0

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
What is the relative mass of a neutron?
A)1/1837 amu
B)½ amu
C)1 amu
D)2 amu
A)1/1837 amu
B)½ amu
C)1 amu
D)2 amu

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
What is the relative electrical charge of an electron?
A)-1
B)+1
C)-2
D)0
A)-1
B)+1
C)-2
D)0

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
Which subatomic particle(s)was not part of the Thomson model of the atom?
A)Proton
B)Neutron
C)Electron
D)Both,choices A and B
A)Proton
B)Neutron
C)Electron
D)Both,choices A and B

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
Which is not part of Dalton's atomic model?
A)Elements are composed of minute,indivisible particles called atoms.
B)Atoms of the same element are alike in mass.
C)Atoms of the same element can be different in size.
D)Chemical compounds are composed of two or more atoms of different elements.
A)Elements are composed of minute,indivisible particles called atoms.
B)Atoms of the same element are alike in mass.
C)Atoms of the same element can be different in size.
D)Chemical compounds are composed of two or more atoms of different elements.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
As the distance between two particles with charges that attract one another increases,the force of attraction will
A)increase.
B)decrease.
C)remain the same.
A)increase.
B)decrease.
C)remain the same.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
The mass of a hydrogen atom is 1.673 x 10 -24 g.How many hydrogen atoms are present in a 1.00g sample of hydrogen?
A)1.00 atom
B)1.67 x 10 -24 atoms
C)5.98 x 10 23 atoms
D)1.67 x 10 24 atoms
A)1.00 atom
B)1.67 x 10 -24 atoms
C)5.98 x 10 23 atoms
D)1.67 x 10 24 atoms

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
What charge does a cation possess?
A)Positive
B)Negative
C)Neutral
A)Positive
B)Negative
C)Neutral

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
What is the relative mass of an electron?
A)1/1837 amu
B)½ amu
C)1 amu
D)2 amu
A)1/1837 amu
B)½ amu
C)1 amu
D)2 amu

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
What is the relative mass of a proton?
A)1/1837 amu
B)½ amu
C)1 amu
D)2 amu
A)1/1837 amu
B)½ amu
C)1 amu
D)2 amu

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
Particles with which electrical charges will repel one another?
A)Positive and positive
B)Positive and negative
C)Negative and negative
D)Both choices A and C
A)Positive and positive
B)Positive and negative
C)Negative and negative
D)Both choices A and C

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
Particles with which electric charges will attract one another?
A)Positive and positive
B)Positive and negative
C)Negative and negative
D)Both,choices A and B
A)Positive and positive
B)Positive and negative
C)Negative and negative
D)Both,choices A and B

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
What charge does an anion possess?
A)Positive
B)Negative
C)Neutral
A)Positive
B)Negative
C)Neutral

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
What is the atomic number of an atom that contains 9 protons,9 electrons,and 10 neutrons?
A)9
B)10
C)18
D)19
A)9
B)10
C)18
D)19

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
Which subatomic particle contributes least to the mass of the nucleus?
A)Proton
B)Neutron
C)Electron
A)Proton
B)Neutron
C)Electron

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
The combined number of protons and neutrons in an atom is known as its
A)atomic number.
B)mass number.
C)atomic mass.
D)molecular mass.
A)atomic number.
B)mass number.
C)atomic mass.
D)molecular mass.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
An isotope of sodium has the atomic number 11 and the mass number 23.An atom of this isotope contains
A)12 electrons
B)12 protons
C)12 neutrons
D)23 electrons
A)12 electrons
B)12 protons
C)12 neutrons
D)23 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
One isotope of carbon has the atomic number 6 and the mass number 14.An atom of this isotope contains
A)8 protons
B)14 neutrons
C)6 neutrons
D)6 electrons
A)8 protons
B)14 neutrons
C)6 neutrons
D)6 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
Naturally occurring neon exists as three isotopes.90.51% is Ne-20 with a mass of 19.99 amu,0.27% is Ne-21 with a mass of 20.99 amu,and 9.22% is Ne-22 with a mass of 21.99 amu.What is the atomic mass of neon?
A)10.00 amu
B)20.18 amu
C)20.99 amu
D)62.97 amu
A)10.00 amu
B)20.18 amu
C)20.99 amu
D)62.97 amu

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
Atoms of isotopes of an element contain the same number of
A)protons.
B)neutrons.
C)alpha particles.
A)protons.
B)neutrons.
C)alpha particles.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
The electrical charge of an atom is
A)positive.
B)negative.
C)the atom has no charge,it is neutral.
A)positive.
B)negative.
C)the atom has no charge,it is neutral.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
Atoms of isotopes of an element contain different numbers of
A)protons.
B)neutrons.
C)electrons.
A)protons.
B)neutrons.
C)electrons.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
In isotopic notation atomic number is represented by the subscript
A)A
B)N
C)P
D)Z
A)A
B)N
C)P
D)Z

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
One isotope of oxygen has the atomic number 8 and the mass number 18.An atom of this isotope contains
A)8 neutrons
B)10 electrons
C)8 protons
D)18 electrons
A)8 neutrons
B)10 electrons
C)8 protons
D)18 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
Different isotopes of an element are atoms of that element which have
A)the same atomic number and the same mass number.
B)the same atomic number and different mass number.
C)different atomic number and the same mass number.
D)different atomic number and different mass number.
A)the same atomic number and the same mass number.
B)the same atomic number and different mass number.
C)different atomic number and the same mass number.
D)different atomic number and different mass number.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
Which particle contributes most to the electrical charge of the nucleus?
A)Proton
B)Neutron
C)Electron
A)Proton
B)Neutron
C)Electron

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
In isotopic notation mass number is represented by the superscript
A)A
B)N
C)P
D)Z
A)A
B)N
C)P
D)Z

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
Which subatomic particle contributes least to the mass of an atom?
A)Proton
B)Neutron
C)Electron
A)Proton
B)Neutron
C)Electron

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
Naturally occurring silicon exists as three isotopes.92.23% is Si-28 with a mass of 27.977 amu,4.67% is Si- 29 with a mass of 28.977 amu,and 3.10% is Si-30 with a mass of 29.974 amu.What is the atomic mass of silicon?
A)14.00 amu
B)28.09 amu
C)28.98 amu
D)86.93 amu
A)14.00 amu
B)28.09 amu
C)28.98 amu
D)86.93 amu

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
Which subatomic particle is not found in the nucleus of the atom?
A)Proton
B)Neutron
C)Electron
A)Proton
B)Neutron
C)Electron

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
The mass of a copper atom is 1.045 x 10 -22 g.How many copper atoms are present in a 94.5g sample of copper?
A)9.04 x 10 23
B)1.045 x 10 -22
C)1870
D)94.5
A)9.04 x 10 23
B)1.045 x 10 -22
C)1870
D)94.5

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
The number of protons in an atom is known as its
A)atomic Mass.
B)atomic number.
C)mass number.
D)molecular mass.
A)atomic Mass.
B)atomic number.
C)mass number.
D)molecular mass.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
The electrical charge of a nucleus is
A)positive.
B)negative.
C)the nucleus has no charge,it is neutral.
A)positive.
B)negative.
C)the nucleus has no charge,it is neutral.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
Which isotope has the same number of protons,neutrons,and electrons?
A)Cl-35
B)Cl-37
C)P-31
D)S-32
A)Cl-35
B)Cl-37
C)P-31
D)S-32

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
How many electrons are in a neutral atom of Ar-40?
A)18
B)22
C)40
D)58
A)18
B)22
C)40
D)58

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
What is the atomic number of an atom that contains 40 protons,40 electrons,and 51neutrons?
A)11
B)40
C)51
D)91
A)11
B)40
C)51
D)91

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
The number of protons and neutrons in an atom are added to find the atom's
A)atomic number.
B)ionic charge.
C)number of electrons.
D)mass number.
A)atomic number.
B)ionic charge.
C)number of electrons.
D)mass number.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
The average mass of an atom of sulfur is 5.33 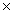 10 -23 g.How many sulfur atoms are present in a 100.0g sample of sulfur?
10 -23 g.How many sulfur atoms are present in a 100.0g sample of sulfur?
A)5.33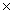 10 -21
10 -21
B)1.88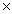 10 -21
10 -21
C)5.33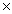 10 24
10 24
D)1.88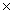 10 24
10 24
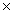 10 -23 g.How many sulfur atoms are present in a 100.0g sample of sulfur?
10 -23 g.How many sulfur atoms are present in a 100.0g sample of sulfur?A)5.33
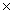 10 -21
10 -21B)1.88
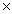 10 -21
10 -21C)5.33
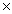 10 24
10 24D)1.88
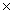 10 24
10 24
Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
A substance has 13 protons,14 neutrons,and 13 electrons.Which isotope is it?
A)Al-13
B)Al-14
C)Al-27
D)Al-40
A)Al-13
B)Al-14
C)Al-27
D)Al-40

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
The element with atomic number 53 is
A)Iron
B)Iridium
C)Iodine
D)Indium
A)Iron
B)Iridium
C)Iodine
D)Indium

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
What is the atomic number of sodium?
A)11
B)16
C)23
D)32
A)11
B)16
C)23
D)32

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
What is the atomic number of magnesium?
A)12
B)24
C)25
D)55
A)12
B)24
C)25
D)55

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
Which contains the largest number of neutrons?
A)C-12
B)P-31
C)Br-80
D)Sr-90
A)C-12
B)P-31
C)Br-80
D)Sr-90

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
The average mass of one atom of carbon is 2.00 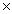 10 -23 g.How many carbon atoms are present in a 40.0g sample of carbon?
10 -23 g.How many carbon atoms are present in a 40.0g sample of carbon?
A)7.96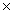 10 -22
10 -22
B)4.98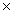 10 -25
10 -25
C)2.00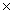 10 24
10 24
D)2.06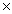 10 4
10 4
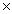 10 -23 g.How many carbon atoms are present in a 40.0g sample of carbon?
10 -23 g.How many carbon atoms are present in a 40.0g sample of carbon?A)7.96
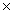 10 -22
10 -22B)4.98
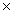 10 -25
10 -25C)2.00
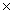 10 24
10 24D)2.06
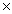 10 4
10 4
Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
The concept that most of the atom's mass is concentrated in a small nucleus surrounded by electrons was the contribution of
A)Dalton
B)Thomson
C)Rutherford
D)Chadwick
A)Dalton
B)Thomson
C)Rutherford
D)Chadwick

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
Models of the atom were proposed by Rutherford,Dalton,and Thomson.Which choice places them in the correct chronological order of their appearance?
A)Rutherford,Dalton,Thomson
B)Thomson,Rutherford,Dalton
C)Dalton,Rutherford,Thomson
D)Dalton,Thomson,Rutherford
A)Rutherford,Dalton,Thomson
B)Thomson,Rutherford,Dalton
C)Dalton,Rutherford,Thomson
D)Dalton,Thomson,Rutherford

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
What is the mass number of an atom that contains 30 protons,30 electrons,and 35 neutrons?
A)5
B)30
C)65
D)95
A)5
B)30
C)65
D)95

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
Which isotope contains the same number of protons,neutrons,and electrons?
A)H-1
B)Na-23
C)C-12
D)Al-27
A)H-1
B)Na-23
C)C-12
D)Al-27

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
Which contains the fewest neutrons?
A)H-2
B)H-3
C)He-4
D)He-5
A)H-2
B)H-3
C)He-4
D)He-5

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
What is the number of neutrons in a neutral atom of Ar-40?
A)18
B)22
C)40
D)58
A)18
B)22
C)40
D)58

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
What is the mass number of an atom that contains 23 protons,23 electrons,and 28 neutrons?
A)5
B)28
C)51
D)74
A)5
B)28
C)51
D)74

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
The element with atomic number 27 is
A)Carbon
B)Copper
C)Cobalt
D)Calcium
A)Carbon
B)Copper
C)Cobalt
D)Calcium

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
How many protons are in a neutral atom of Ar-40?
A)18
B)22
C)40
D)58
A)18
B)22
C)40
D)58

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
Ions can be formed from atoms by losing or gaining electrons.Select the alternative that states the correct number of protons,neutrons,and electrons in 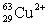 .
.
A)29 protons,34 neutrons,31 electrons
B)29 protons,34 neutrons,27 electrons
C)27 protons,29 neutrons,29 electrons
D)29 protons,63 neutrons,29 electrons
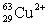 .
.A)29 protons,34 neutrons,31 electrons
B)29 protons,34 neutrons,27 electrons
C)27 protons,29 neutrons,29 electrons
D)29 protons,63 neutrons,29 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
Based on the following information,calculate the mass of one atom of 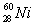 . mass of an electron = 9.110 x 10-28 g
. mass of an electron = 9.110 x 10-28 g
Mass of a proton = 1.673 x 10-24 g
Mass of a neutron = 1.675 x 10-24 g
A)58.69 g
B)1.474 x 10-22 g
C)9.746 x 10-23 g
D)1.005 x 10-22 g
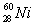 . mass of an electron = 9.110 x 10-28 g
. mass of an electron = 9.110 x 10-28 gMass of a proton = 1.673 x 10-24 g
Mass of a neutron = 1.675 x 10-24 g
A)58.69 g
B)1.474 x 10-22 g
C)9.746 x 10-23 g
D)1.005 x 10-22 g

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
The number of protons in an atom of boron-11 is
A)4
B)5
C)6
D)7
A)4
B)5
C)6
D)7

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
The number of electrons in an atom of iron-56 is
A)26
B)30
C)53
D)3
A)26
B)30
C)53
D)3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
Atom A has 5 protons and 6 neutrons;atom B has 6 protons and 5 neutrons.These atoms are
A)isotopes of the same element.
B)isomers of the same element.
C)atoms of different elements.
D)identical in physical properties.
A)isotopes of the same element.
B)isomers of the same element.
C)atoms of different elements.
D)identical in physical properties.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
Ions can be formed from atoms by losing or gaining electrons.Select the alternative that states the correct number of protons,neutrons,and electrons in 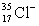 .
.
A)17 protons,18 neutrons,16 electrons
B)17 protons,18 neutrons,18 electrons
C)18 protons,17 neutrons,19 electrons
D)17 protons,36 neutrons,17 electrons
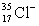 .
.A)17 protons,18 neutrons,16 electrons
B)17 protons,18 neutrons,18 electrons
C)18 protons,17 neutrons,19 electrons
D)17 protons,36 neutrons,17 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is not supported by Dalton's Atomic Theory?
A)atoms
B)isotopes
C)compounds
D)elements
A)atoms
B)isotopes
C)compounds
D)elements

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
The atomic mass of an element is
A)the mass of the most abundant isotope of that element.
B)the weighted average of the masses of the naturally occurring isotopes of that element.
C)the arithmetic average of the masses of the isotopes of that element.
D)the ratio of the mass of one atom of an isotope of that element to the mass of hydrogen.
A)the mass of the most abundant isotope of that element.
B)the weighted average of the masses of the naturally occurring isotopes of that element.
C)the arithmetic average of the masses of the isotopes of that element.
D)the ratio of the mass of one atom of an isotope of that element to the mass of hydrogen.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
Based on the following information,calculate the mass of one atom of 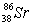 . mass of an electron = 9.110 x 10-28 g
. mass of an electron = 9.110 x 10-28 g
Mass of a proton = 1.673 x 10-24 g
Mass of a neutron = 1.675 x 10-24 g
A)1.440 x 10-22 g
B)87.62 g
C)2.077 x 10-22 g
D)1.455 x 10-22 g
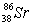 . mass of an electron = 9.110 x 10-28 g
. mass of an electron = 9.110 x 10-28 gMass of a proton = 1.673 x 10-24 g
Mass of a neutron = 1.675 x 10-24 g
A)1.440 x 10-22 g
B)87.62 g
C)2.077 x 10-22 g
D)1.455 x 10-22 g

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
Which experiment led to the notion that the atom contains an extremely small,positively charged nucleus?
A)Millikan's oil drop experiment
B)Rutherford's gold foil experiment
C)Thomson's cathode ray experiment
D)Dalton's atomic experiment
A)Millikan's oil drop experiment
B)Rutherford's gold foil experiment
C)Thomson's cathode ray experiment
D)Dalton's atomic experiment

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
The number of electrons in an atom of phosphorous-31 is
A)9
B)15
C)16
D)22
A)9
B)15
C)16
D)22

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
Let Z represent atomic number,A represent mass number,and N represent the number of neutrons in an atom.Which of the following is correct?
A)N = A + Z
B)Z = A + N
C)N = A - Z
D)A = N - Z
A)N = A + Z
B)Z = A + N
C)N = A - Z
D)A = N - Z

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
Ca-40,K-39,and Sc-41 all have the same
A)atomic mass.
B)atomic number.
C)number of electrons.
D)number of neutrons.
A)atomic mass.
B)atomic number.
C)number of electrons.
D)number of neutrons.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
The Law of Definite proportions pertains to
A)compounds composed of elements.
B)mixtures composed of compounds.
C)compounds composed of mixtures.
D)mixtures composed of elements.
A)compounds composed of elements.
B)mixtures composed of compounds.
C)compounds composed of mixtures.
D)mixtures composed of elements.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
Ions can be formed from atoms by losing or gaining electrons.Select the alternative that states the correct number of protons,neutrons,and electrons in 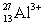 .
.
A)13 protons,14 neutrons,and 16 electrons
B)13 protons,14 neutrons,and 10 electrons
C)10 protons,17 neutrons,13 electrons
D)13 protons,27 neutrons,10 electrons
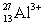 .
.A)13 protons,14 neutrons,and 16 electrons
B)13 protons,14 neutrons,and 10 electrons
C)10 protons,17 neutrons,13 electrons
D)13 protons,27 neutrons,10 electrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
The name of the isotope containing one proton and two neutrons is
A)protium.
B)deuterium.
C)tritium.
D)helium.
A)protium.
B)deuterium.
C)tritium.
D)helium.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
Isotopes of an element always have the same
A)mass number.
B)number of neutrons.
C)number of subatomic particles.
D)atomic number.
A)mass number.
B)number of neutrons.
C)number of subatomic particles.
D)atomic number.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
The number of protons in an atom of sodium-23 is
A)11
B)12
C)16
D)23
A)11
B)12
C)16
D)23

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
A substance has 15 protons,16 neutrons,and 15 electrons.Which isotope is it?
A)P-30
B)P-31
C)S-30
D)S-31
A)P-30
B)P-31
C)S-30
D)S-31

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
The element with atomic number 53 always contains
A)53 neutrons
B)53 protons
C)26 neutrons and 27 protons
D)26 protons and 27 neutrons
A)53 neutrons
B)53 protons
C)26 neutrons and 27 protons
D)26 protons and 27 neutrons

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


