Deck 3: Electronic Structure and the Periodic Law
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 3: Electronic Structure and the Periodic Law
1
The total number of unpaired electrons in silicon,Si,is _____ .
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3
2
2
Which of the following elements has the electronic configuration shown here?
1s2 2s2 2p6 3s2 3p1
A) F
B) Al
C) Mg
D) Ga
1s2 2s2 2p6 3s2 3p1
A) F
B) Al
C) Mg
D) Ga
Al
3
Suppose an electron moved from the second shell to the third shell.
A) The move required an input of energy.
B) The move gave off energy.
C) Electrons can move spontaneously from shell to shell.
D) Electrons can't move from shell to shell,but can move into the nucleus.
A) The move required an input of energy.
B) The move gave off energy.
C) Electrons can move spontaneously from shell to shell.
D) Electrons can't move from shell to shell,but can move into the nucleus.
The move required an input of energy.
4
If the formula for sulfuric acid is H2SO4,what would be the expected formula for the compound between hydrogen and tellurium,Te?
A) H2TeO4
B) HTe
C) HTe2
D) H2Te
A) H2TeO4
B) HTe
C) HTe2
D) H2Te

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
What is the symbol of the element that is in Period 4,and Group IIA(2)of the periodic table?
A) Zn
B) Ca
C) C
D) Ti
A) Zn
B) Ca
C) C
D) Ti

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
How many electrons are in the outer shell of element 15?
A) 5
B) 3
C) 15
D) 2
A) 5
B) 3
C) 15
D) 2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following has the same number of outer shell electrons as sulfur,S?
A) C
B) O
C) N
D) F
A) C
B) O
C) N
D) F

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following subshells has the higher energy?
A) 4s
B) 4p
C) 4d
D) 4f
A) 4s
B) 4p
C) 4d
D) 4f

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements applies to p subshells?
A) The p subshell can contain a maximum of 14 electrons.
B) The p subshell is subdivided into three perpendicular shapes.
C) The p subshell fills with 2 electrons in px,then 2 in py then 2 in pz.
D) All of these statements are correct with reference to p subshells.
A) The p subshell can contain a maximum of 14 electrons.
B) The p subshell is subdivided into three perpendicular shapes.
C) The p subshell fills with 2 electrons in px,then 2 in py then 2 in pz.
D) All of these statements are correct with reference to p subshells.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
What is the shell number for the outer shell electrons in bromine,Br?
A) 4
B) 3
C) 5
D) 6
A) 4
B) 3
C) 5
D) 6

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
What is the maximum number of electrons that can occupy the third shell?
A) 2
B) 10
C) 18
D) 32
A) 2
B) 10
C) 18
D) 32

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following contains the same total number of unpaired electrons as potassium (K)?
A) Sc
B) Cl
C) Mg
D) more than one response is correct
A) Sc
B) Cl
C) Mg
D) more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following elements should have properties similar to those of nitrogen (element 7)?
A) C
B) Si
C) P
D) O
A) C
B) Si
C) P
D) O

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following elements is found in the same period of the periodic table as tin?
A) silicone
B) arsenic
C) tellurium
D) technetium
A) silicone
B) arsenic
C) tellurium
D) technetium

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following will not have electrons in the 3rd orbital?
A) K
B) Mg
C) Na+
D) Ag+
A) K
B) Mg
C) Na+
D) Ag+

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
Which element is the first one in the group IVA(14)of the periodic table?
A) C
B) Na
C) Sc
D) Ti
A) C
B) Na
C) Sc
D) Ti

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
The maximum number of electrons that can occupy a 3d subshell is _____ .
A) 2
B) 4
C) 6
D) 10
A) 2
B) 4
C) 6
D) 10

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
The maximum number of electrons that can occupy a 4p orbital is _____ .
A) 2
B) 4
C) 6
D) 10
A) 2
B) 4
C) 6
D) 10

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
The total number of f orbitals in an f subshell is _____ .
A) 2
B) 5
C) 7
D) 10
A) 2
B) 5
C) 7
D) 10

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
Silver,Ag,belongs to what period of the periodic table?
A) 1
B) 2
C) 5
D) 12
A) 1
B) 2
C) 5
D) 12

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following elements is most likely to be a metalloid?
A) Ge
B) Mo
C) S
D) Cs
A) Ge
B) Mo
C) S
D) Cs

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
Which element is represented by the distinguishing electron configuration 5d 9?
A) Pd
B) Ag
C) Pt
D) Au
A) Pd
B) Ag
C) Pt
D) Au

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following elements contains a total of 10 "s" electrons?
A) Sr
B) Sb
C) Cs
D) more than one response is correct
A) Sr
B) Sb
C) Cs
D) more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
One mole of an element would weigh the same as a mole of an isotope of the same element.
A) Mg
B) Be
C) Al
D) B
A) Mg
B) Be
C) Al
D) B

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
The electronic configuration of element 17 ends with _____ .
A) 2p 3
B) 3p 5
C) 2p 5
D) 3p 3
A) 2p 3
B) 3p 5
C) 2p 5
D) 3p 3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
The radius of a K atom is ____ a Ca atom.
A) smaller than
B) larger than
C) equal to
D) inverted from
A) smaller than
B) larger than
C) equal to
D) inverted from

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
Which element has the distinguishing electron,5p4?
A) Br
B) Mn
C) Te
D) Kr
A) Br
B) Mn
C) Te
D) Kr

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
Metalloids can express the characteristics of both metals and nonmetals.Which of the elements below is more likely to have the characteristics of a metal than a nonmetal?
A) As
B) Se
C) Si
D) Sb
A) As
B) Se
C) Si
D) Sb

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
The melting point of oxygen is ____ selenium,Se.
A) lower than
B) higher than
C) equal to
D) inverted from
A) lower than
B) higher than
C) equal to
D) inverted from

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
What type of electron is the distinguishing electron in S?
A) s
B) p
C) d
D) f
A) s
B) p
C) d
D) f

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following elements is a nonmetal?
A) Ni
B) Cu
C) Ba
D) I
A) Ni
B) Cu
C) Ba
D) I

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
In which of the following elements does the distinguishing electron occupy a d subshell?
A) Cr
B) O
C) Kr
D) Sr
A) Cr
B) O
C) Kr
D) Sr

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a noble gas?
A) gold
B) platinum
C) neon
D) chlorine
A) gold
B) platinum
C) neon
D) chlorine

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following elements is classified as a representative metal?
A) element 30
B) element 26
C) element 38
D) element 63
A) element 30
B) element 26
C) element 38
D) element 63

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following distinguishing electrons represents an element with properties similar to an element with a 3p 3 distinguishing electron?
A) 4p 3
B) 3p 2
C) 3d 3
D) 2p 2
A) 4p 3
B) 3p 2
C) 3d 3
D) 2p 2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements conforms to the trends within the Periodic Table of the Elements?
A) Elements become more likely to be gases at the bottom of a group.
B) Elements become more likely to be a solid at the bottom of a group.
C) Elements become more likely to be darkly colored at the bottom of a group.
D) Elements become more likely to be more brittle at the bottom of a group.
A) Elements become more likely to be gases at the bottom of a group.
B) Elements become more likely to be a solid at the bottom of a group.
C) Elements become more likely to be darkly colored at the bottom of a group.
D) Elements become more likely to be more brittle at the bottom of a group.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following pairs have the same number of electrons in the outermost shell?
A) K and Ca
B) S4+ and Al3+
C) Na+ and Ne
D) Ne and He
A) K and Ca
B) S4+ and Al3+
C) Na+ and Ne
D) Ne and He

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of these elements is a gas at room temperature?
A) antimony
B) phosphorus
C) nitrogen
D) arsenic
A) antimony
B) phosphorus
C) nitrogen
D) arsenic

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following elements is classified as a transition element?
A) element 76
B) element 60
C) element 56
D) element 17
A) element 76
B) element 60
C) element 56
D) element 17

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
Which element is used for relieving the symptoms of a cold?
A) Au
B) K
C) Zn
D) Fe
A) Au
B) K
C) Zn
D) Fe

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
For which of the listed elements (E)would the energy required for the following reaction be the least? E(g) E+(g)+ e-
A) Ga
B) Na
C) Ca
D) K
A) Ga
B) Na
C) Ca
D) K

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
Choose the correct electronic configuration for the element As.
A) [Ar] 4s2 3d10 4p3
B) 4s2 3d10 4p3
C) [Ar] 4s2 4d10 4p3
D) 4s2 4d10 4p3
A) [Ar] 4s2 3d10 4p3
B) 4s2 3d10 4p3
C) [Ar] 4s2 4d10 4p3
D) 4s2 4d10 4p3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
The family name for group VIIIA(18)of the periodic table is
A) transition metals.
B) noble gases.
C) representative elements.
D) There is no family name
A) transition metals.
B) noble gases.
C) representative elements.
D) There is no family name

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
The element chlorine is found in the same group of the periodic table as _____ .
A) I
B) F
C) Br
D) all of these
A) I
B) F
C) Br
D) all of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is a representation of a p orbital?
A)

B)
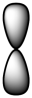
C)
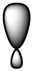
D)
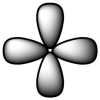
A)

B)
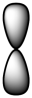
C)
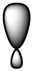
D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the correct designation for an electron occupying the orbital shown below? 
A) 2p
B) 3s
C) 3d
D) 4f

A) 2p
B) 3s
C) 3d
D) 4f

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following correctly arranges the given subshells in order of increasing energy (lowest to highest)?
A) 2s < 3d < 4s
B) 4s < 3p < 3s
C) 2s < 3d < 4p
D) 4p < 4f < 5s
A) 2s < 3d < 4s
B) 4s < 3p < 3s
C) 2s < 3d < 4p
D) 4p < 4f < 5s

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following metals (M)would produce the fastest reaction with ethyl alcohol (C2H5OH)as shown? 2M + 2C2H5OH 2C2H5OM + H2
A) K
B) Li
C) Sr
D) Al
A) K
B) Li
C) Sr
D) Al

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
____ explains why orbitals can contain a maximum of two electrons.
A) Hund's rule
B) Pauli exclusion principle
C) Periodic law
D) None of these
A) Hund's rule
B) Pauli exclusion principle
C) Periodic law
D) None of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
The highest-energy shell of an element that contains electrons is know as the _____ .
A) subshell
B) atomic orbital
C) valence shell
D) none of these
A) subshell
B) atomic orbital
C) valence shell
D) none of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
Which pair of the following represents the same electron configuration?
A) [Ne] 3s2 and 1s2 2s2 2p6 3s2
B) [He] 3s2 and 1s2 2s2 2p6 3s2
C) [Ar]3s2 and 1s2 2s2 2p6 3s2
D) All represent the same configuration.
A) [Ne] 3s2 and 1s2 2s2 2p6 3s2
B) [He] 3s2 and 1s2 2s2 2p6 3s2
C) [Ar]3s2 and 1s2 2s2 2p6 3s2
D) All represent the same configuration.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Which element has the largest atomic radii?
A) I
B) F
C) Li
D) Cs
A) I
B) F
C) Li
D) Cs

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
Unlike the original attempt by Mendeleev,the modern periodic table arranges elements based on what elemental feature?
A) mass number
B) atomic number
C) isotope composition
D) relative size
A) mass number
B) atomic number
C) isotope composition
D) relative size

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
What is the basis for placing groups of elements into a family on the periodic table?
A) similar atomic mass numbers
B) identical chemical and physical properties
C) similar colors,shapes and uses
D) similar valence electron configurations
A) similar atomic mass numbers
B) identical chemical and physical properties
C) similar colors,shapes and uses
D) similar valence electron configurations

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the correct valence electron configuration of phosphorus?
A)
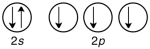
B)
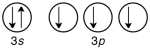
C)
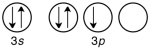
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
What rule,principle or law is used to explain that an electrons will not share the same orbital if there is an empty orbital of the same energy available?
A) Aufbau principle
B) Hund's rule
C) Pauli principle
D) Avogadro's law
A) Aufbau principle
B) Hund's rule
C) Pauli principle
D) Avogadro's law

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a reasonable explanation as to why atomic radii become smaller as one moves to the right for the representative elements in a period?
A) The charge of the nucleus increases,pulling the shells closer.
B) More densely packed shells are always smaller.
C) Elements become less metallic.
D) Atomic radii actually increase as you move to the right.
A) The charge of the nucleus increases,pulling the shells closer.
B) More densely packed shells are always smaller.
C) Elements become less metallic.
D) Atomic radii actually increase as you move to the right.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
____ states that,electrons will not join other electrons in an orbital if an empty orbital of the same energy is available.
A) Hund's rule
B) Pauli exclusion principle
C) Periodic law
D) None of these
A) Hund's rule
B) Pauli exclusion principle
C) Periodic law
D) None of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is considered a transition element?
A) tungsten
B) holmium
C) radon
D) cesium
A) tungsten
B) holmium
C) radon
D) cesium

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
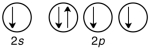
Which of the following violates the Pauli exclusion principle?
A)
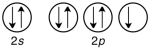
B)
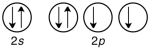
C)
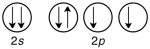
D) All of them
A)

B)

C)

D) All of them

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
Sodium,Na,and potassium,K,are in the same period.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
The distinguishing electron for most non-metals is an s electron.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
A bromine atom has a larger radius than a chlorine atom.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
All non-metals are representative elements.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
The radius of a magnesium atom is larger than the radius of a sodium atom.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
The fourth shell contains four subshells.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
Tin (Sn)is a representative element.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
Hydrogen is a noble gas.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
Argon completes the fourth period of the periodic table.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
A 3d orbital can hold 6 electrons.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
The magnesium atom loses an electron easier than a calcium atom.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
The distinguishing electron in antimony,Sb,is found in a 5p subshell.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
All of the transition elements are metals.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
The electron configuration for each shell starts with an s subshell.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
Calcium citrate,Ca3(C6H5O7)2,is commonly used in the production of dietary calcium supplements.How many representative elements are found in calcium citrate?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
An anion is smaller than the atom from which it is produced.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
The second shell has a maximum capacity of 6 electrons.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
Element number 92,uranium,U,is a metal.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
Tin (Sn),which is found in Group IVA,has four electrons in the outside shell.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
The fifth period begins with Rb.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


