Deck 14: Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 14: Aldehydes and Ketones
1
Which of the following is the structure for acetone?
A)
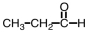
B)
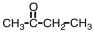
C)
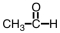
D)
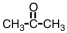
A)
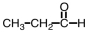
B)
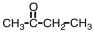
C)
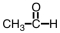
D)
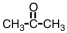
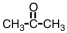
2
What structural characteristic is shared by the aldehydes and the ketones?
A) They both are straight chain compounds.
B) Both of these compound classes have as the smallest compound a 5 carbon skeleton.
C) Aldehydes and ketones both contain a carbonyl carbon.
D) Aldehydes and ketones have no shared characteristics.
A) They both are straight chain compounds.
B) Both of these compound classes have as the smallest compound a 5 carbon skeleton.
C) Aldehydes and ketones both contain a carbonyl carbon.
D) Aldehydes and ketones have no shared characteristics.
Aldehydes and ketones both contain a carbonyl carbon.
3
Which of the following pure compounds can exhibit hydrogen bonding with itself?
A)
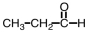
B)
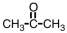
C)
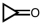
D) none of these
A)
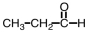
B)
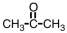
C)

D) none of these
none of these
4
Which of the following will react with Tollens' reagent?
A)
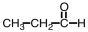
B)
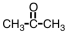
C)
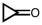
D)
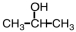
A)
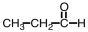
B)
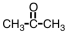
C)

D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds can form hydrogen bonds with water molecules?
A)
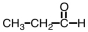
B)
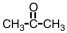
C)
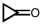
D) all of them
A)
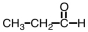
B)
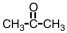
C)

D) all of them

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
The name 4,5-dichlorocyclohexanone should be
A) 1,2-dichlorocyclohexanone.
B) 2,3-dichlorocyclohexanone.
C) 3,4-dichlorocyclohexanone.
D) 4,5-dichlorocyclohexanone.
A) 1,2-dichlorocyclohexanone.
B) 2,3-dichlorocyclohexanone.
C) 3,4-dichlorocyclohexanone.
D) 4,5-dichlorocyclohexanone.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a requirement of Tollens' reagent?
A) The reaction must be run in a flask.
B) The glassware should be grease-free.
C) An air source must be present to dry the results before interpretation.
D) The reaction must be run at the boiling point of the substance being tested.
A) The reaction must be run in a flask.
B) The glassware should be grease-free.
C) An air source must be present to dry the results before interpretation.
D) The reaction must be run at the boiling point of the substance being tested.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name for the following compound? 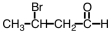
A) 3-bromobutanal
B) 2-bromobutanal
C) 3-bromobutanone
D) 2-bromobutanone
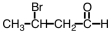
A) 3-bromobutanal
B) 2-bromobutanal
C) 3-bromobutanone
D) 2-bromobutanone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
What is the group that distinguishes aldehydes from most other classes of compounds?
A) carboxyl
B) carbonyl
C) hydroxy
D) amide
A) carboxyl
B) carbonyl
C) hydroxy
D) amide

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name for compound shown below? 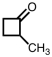
A) methylbutanone
B) 2-methylcyclobutanone
C) 1-methylcyclobutanal
D) 1-methylcyclobutanone

A) methylbutanone
B) 2-methylcyclobutanone
C) 1-methylcyclobutanal
D) 1-methylcyclobutanone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
Which is a test for the presence of an aldehyde?
A) Silver's reagent
B) boiling point determination
C) Benedict's reagent
D) a color change at boiling point
A) Silver's reagent
B) boiling point determination
C) Benedict's reagent
D) a color change at boiling point

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
What is the reason for norethynodrel's ability to function as a contraceptive?
A) It is a ketone that is required to neutralize ovum production.
B) It is capable of mimicking progesterone establishing a false pregnancy.
C) It replaces estrogen and displaces the normal hormonal control.
D) It bonds to estrogen to reduce the estrogen levels.
A) It is a ketone that is required to neutralize ovum production.
B) It is capable of mimicking progesterone establishing a false pregnancy.
C) It replaces estrogen and displaces the normal hormonal control.
D) It bonds to estrogen to reduce the estrogen levels.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
What are the risks of constant sunbathing?
A) skin cancer
B) premature aging of the skin
C) thickening of the skin
D) more than one response is correct
A) skin cancer
B) premature aging of the skin
C) thickening of the skin
D) more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
In the IUPAC nomenclature system,the name of which of the following would end in "-al"?
A) an alcohol
B) an aldehyde
C) an alkane
D) a phenol
A) an alcohol
B) an aldehyde
C) an alkane
D) a phenol

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following products is formed when hydrogen is reacted with 3-methyl-2-butanone?
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) acetal
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) acetal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name for the compound shown below? 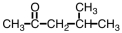
A) 2-methyl-2-pentanone
B) 2-methyl-4-pentanone
C) 4-methyl-2-pentanone
D) 2-methol-4-pentanol
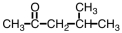
A) 2-methyl-2-pentanone
B) 2-methyl-4-pentanone
C) 4-methyl-2-pentanone
D) 2-methol-4-pentanol

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
Assuming that molecular weights are comparable,which of the following compounds would you expect to be the highest boiling?
A) aldehyde
B) alkene
C) alcohol
D) ketone
A) aldehyde
B) alkene
C) alcohol
D) ketone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
A positive Benedict's test is indicated by the formation of which of the following?
A) Cu2O
B) Cu
C) Cu2+
D) metallic mirror
A) Cu2O
B) Cu
C) Cu2+
D) metallic mirror

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
To what characteristic(s)do aldehydes and ketones owe their ability to form hydrogen bonds?
A) oxygen is always polar
B) single bound oxygen
C) having a doubly bonded oxygen
D) all of the responses are correct
A) oxygen is always polar
B) single bound oxygen
C) having a doubly bonded oxygen
D) all of the responses are correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
What is the consequence of the ability of aldehydes and ketones to form hydrogen bonds?
A) They are both highly colored when in the solid state.
B) They both have boiling points less than the comparable weight alcohol.
C) They prefer to hydrogen bond molecules of the same formula and will not dissolve well in water.
D) There is more than one correct response.
A) They are both highly colored when in the solid state.
B) They both have boiling points less than the comparable weight alcohol.
C) They prefer to hydrogen bond molecules of the same formula and will not dissolve well in water.
D) There is more than one correct response.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds upon hydrolysis would yield CH3CH2OH and CH3CH2CHO?
A)
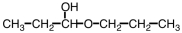
B)
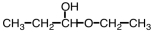
C)
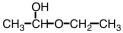
D)
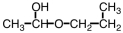
A)
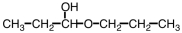
B)
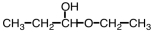
C)
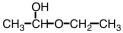
D)
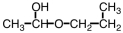

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
Hemiacetals are
A) partial acetals that can be converted by a reduction reaction.
B) unstable compounds that convert to acetals in the presence of an alcohol and an acid.
C) semiacetals produced by alcohol subtraction from a ketone.
D) There is more than one correct response.
A) partial acetals that can be converted by a reduction reaction.
B) unstable compounds that convert to acetals in the presence of an alcohol and an acid.
C) semiacetals produced by alcohol subtraction from a ketone.
D) There is more than one correct response.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
Overdose of ____ during pregnancy has proven to cause birth defects.
A) Vitamin A
B) Vitamin B6
C) Vitamin C
D) Vitamin D
A) Vitamin A
B) Vitamin B6
C) Vitamin C
D) Vitamin D

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a water soluble,organic solvent?
A) vanilloids
B) hemiacetals
C) acetone
D) diethyl ether
A) vanilloids
B) hemiacetals
C) acetone
D) diethyl ether

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is an acetal?
A)
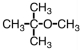
B)
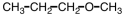
C)
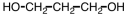
D)
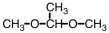
A)

B)
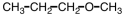
C)
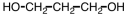
D)
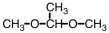

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
What is the result of a hydrolysis reaction?
A) getting the chemicals involved wet
B) causing an oxidation-reduction reaction resulting in hydration
C) splitting of the molecule into the component substances
D) one that results in the formation water
A) getting the chemicals involved wet
B) causing an oxidation-reduction reaction resulting in hydration
C) splitting of the molecule into the component substances
D) one that results in the formation water

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would you expect to be the most flammable?
A) vanillin
B) benzaldehyde
C) camphor
D) acetone
A) vanillin
B) benzaldehyde
C) camphor
D) acetone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is used as a component of the preservative formalin?
A)
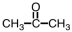
B)
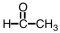
C)
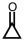
D)
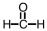
A)
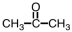
B)
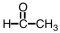
C)
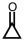
D)
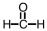

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
Which reaction requires platinum as a catalyst?
A) oxidation of an aldehyde or ketone
B) reduction of an aldehyde or ketone
C) reaction of an aldehyde or ketone with an alcohol
D) All of the responses are reactions that are catalyzed by platinum.
A) oxidation of an aldehyde or ketone
B) reduction of an aldehyde or ketone
C) reaction of an aldehyde or ketone with an alcohol
D) All of the responses are reactions that are catalyzed by platinum.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
What is the flavoring for margarine?
A) biacetyl
B) acetone
C) menthone
D) citral
A) biacetyl
B) acetone
C) menthone
D) citral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is an aldehyde flavoring agent?
A) biacetyl
B) menthol
C) vanillin
D) camphor
A) biacetyl
B) menthol
C) vanillin
D) camphor

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
What is the major difference between a cyclic hemiacetal and a cyclic acetal?
A) The cyclic hemiacetal is an alcohol,whereas the cyclic acetal is an ether.
B) The cyclic hemiacetal is an acid,and the cyclic acetal is a base.
C) The cyclic hemiacetal contains more carbons in the ring than the cyclic acetal.
D) All of the responses are correct.
A) The cyclic hemiacetal is an alcohol,whereas the cyclic acetal is an ether.
B) The cyclic hemiacetal is an acid,and the cyclic acetal is a base.
C) The cyclic hemiacetal contains more carbons in the ring than the cyclic acetal.
D) All of the responses are correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
What starting material is necessary to complete the reaction below? 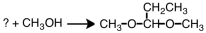
A)
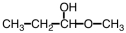
B)
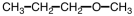
C)
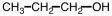
D)
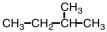

A)
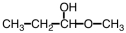
B)
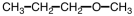
C)
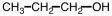
D)
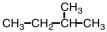

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
____ is the active ingredient in Heet® and Methacin®,and Pain Doctor®,which are pain relieving creams that are over the counter preparation.
A) aspirin
B) resiniferatoxin
C) capsaicin
D) RTX
A) aspirin
B) resiniferatoxin
C) capsaicin
D) RTX

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is,in an injectable form,used for contraception?
A) norethynodrel
B) estrogen
C) progestin
D) testosterone
A) norethynodrel
B) estrogen
C) progestin
D) testosterone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
To what class of compounds does glucose belong?
A) cyclic ketone
B) cyclic aldehyde
C) cyclic hemiacetal
D) carboxylic acid
A) cyclic ketone
B) cyclic aldehyde
C) cyclic hemiacetal
D) carboxylic acid

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is an important organic solvent?
A) menthone
B) acetone
C) citral
D) phenol
A) menthone
B) acetone
C) citral
D) phenol

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
Hemiacetals are
A) produced on the oxidation of a carboxylic acid.
B) result of the reaction between an aldehyde and a ketone.
C) are produced when an ether reacts with a ketone.
D) the unstable product of the oxidation of an aldehyde.
A) produced on the oxidation of a carboxylic acid.
B) result of the reaction between an aldehyde and a ketone.
C) are produced when an ether reacts with a ketone.
D) the unstable product of the oxidation of an aldehyde.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Predict the resultant compound class when an aldehyde is hydrogenated.
A) hemiketal
B) alcohol
C) carboxylic acid
D) no reaction
A) hemiketal
B) alcohol
C) carboxylic acid
D) no reaction

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is most toxic?
A) formaldehyde
B) benzaldehyde
C) acetone
D) camphor
A) formaldehyde
B) benzaldehyde
C) acetone
D) camphor

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
Formaldehyde is a ____ at room temperature.
A) gas
B) liquid
C) solid
D) unknown
A) gas
B) liquid
C) solid
D) unknown

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is NOT a proper IUPAC name?
A) 1-cyclopentanone
B) 1-pentanone
C) 2-pentanone
D) 3-pentanone
A) 1-cyclopentanone
B) 1-pentanone
C) 2-pentanone
D) 3-pentanone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following has the highest boiling point?
A) propanal
B) methanal
C) ethanal
D) butanal
A) propanal
B) methanal
C) ethanal
D) butanal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following substances can be used to produce a chemical tan?
A) benzaldehyde
B) dihydroxyacetone
C) menthone
D) citral
A) benzaldehyde
B) dihydroxyacetone
C) menthone
D) citral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
Norethynodrel is a common drug used for birth control.Which of the following is NOT a common side affect associated with norethynodrel?
A) abdominal bleeding
B) hypertension
C) acne
D) weight loss
A) abdominal bleeding
B) hypertension
C) acne
D) weight loss

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
What is the organic product when a basic solution of Ag(NH3)2+ is added to CH3CH2CH2CHO?
A)
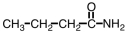
B)
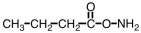
C)
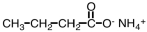
D) No reaction occurs.
A)
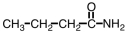
B)
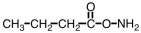
C)
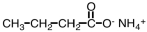
D) No reaction occurs.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
Fully reacting an aldehyde with an alcohol will produce?
A) a carboxylic acid
B) no reaction
C) a primary alcohol
D) an acetal
A) a carboxylic acid
B) no reaction
C) a primary alcohol
D) an acetal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following substances is an integral portion of embalming fluid?
A) methadone
B) methanal
C) ethanol
D) ethanal
A) methadone
B) methanal
C) ethanol
D) ethanal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
What is the common name for 2-butanone?
A) methyl ethyl ketone
B) diethyl ketone
C) isobutyl ketone
D) butyl ketone
A) methyl ethyl ketone
B) diethyl ketone
C) isobutyl ketone
D) butyl ketone

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
Hydrogenation of an aldehyde will produce?
A) a carboxylic acid
B) no reaction
C) a primary alcohol
D) an acetal
A) a carboxylic acid
B) no reaction
C) a primary alcohol
D) an acetal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a condensed structural formula for an aldehyde? (Note: R is used to represent a generic alkyl chain).
A) RCHO
B) RCOH
C) RC(=O)R
D) RCOOH
A) RCHO
B) RCOH
C) RC(=O)R
D) RCOOH

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Our bodies rely on a two step process for detoxification of ethyl alcohol.The first step occurs in the cytoplasm of the liver,where the alcohol is oxidized.Which of the following materials is produced during that step?
A)
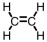
B)
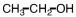
C)
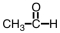
D)
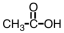
A)

B)
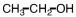
C)
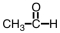
D)
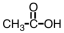

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is an ingredient in some inhalants? It has a characteristic medicinal odor.
A) citral
B) biacetal
C) menthone
D) camphor
A) citral
B) biacetal
C) menthone
D) camphor

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
An unknown compound undergoes no change under oxidizing conditions but reacts readily with hydrogen in the presence of a Pt catalyst.Which of the following might be this unknown compound?
A)
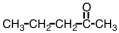
B)
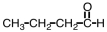
C)
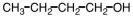
D)
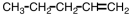
A)
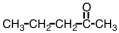
B)
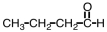
C)
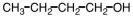
D)
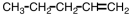

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
Oxidation of an aldehyde will produce?
A) a carboxylic acid
B) no reaction
C) a primary alcohol
D) an acetal
A) a carboxylic acid
B) no reaction
C) a primary alcohol
D) an acetal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following properly ranks the relative aqueous solubility of each functional group?
A) alkene < aldehyde < alcohol < ketone
B) aldehyde < ketone < alkene < alcohol
C) alkene < ketone < alcohol < aldehyde
D) alkene < ketone < aldehyde < alcohol
A) alkene < aldehyde < alcohol < ketone
B) aldehyde < ketone < alkene < alcohol
C) alkene < ketone < alcohol < aldehyde
D) alkene < ketone < aldehyde < alcohol

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a structural representation of benzaldehyde?
A)
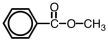
B)
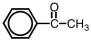
C)
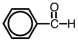
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following will give both a positive Tollens' test and a positive Benedict's test?
A)
B)
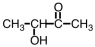
C)
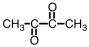
D) All give positive tests for both.
A)

B)

C)

D) All give positive tests for both.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
The intermediate product of two moles of a primary alcohol and one mole of a ketone would be a(n)____.
A) acetal
B) ketal
C) hemiacetal
D) hemiketal
A) acetal
B) ketal
C) hemiacetal
D) hemiketal

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is the substance that causes the symptoms associated with a 'hangover'?
A) methanal
B) 2-octanone
C) ethanal
D) benzaldehyde
A) methanal
B) 2-octanone
C) ethanal
D) benzaldehyde

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
The IUPAC name for formaldehyde is methanal.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
Acetals are prepared from ketones and alcohols.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
Generally,only those organic compounds containing oxygen or nitrogen can hydrogen bond.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
To prepare a ketone,one should oxidize a secondary alcohol.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
Benedict's reagent is a stronger oxidizing reagent than the Tollens' reagent.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
The formation of a ketal from a hemiketal and alcohol is usually a base catalyzed reaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following substances is found in the rinds of lemons,limes and oranges?
A) biacetal
B) dihydroxyacetone
C) menthone
D) citral
A) biacetal
B) dihydroxyacetone
C) menthone
D) citral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
Acetone is the common name for 2-butanone.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
Another name for acetaldehyde is 2-propane.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
The formation of a hemiketal from a ketone and alcohol is a condensation reaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
Aldehydes and ketones would always have a higher boiling point than the corresponding hydrocarbon with the same number of carbon atoms.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
When naming aldehydes,the aldehyde group is at position number 1.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
Acetone can strongly hydrogen bond to water.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
Ketones react readily with the Tollens' reagent.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
Acetone can strongly hydrogen bond to itself.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
The addition of an alcohol molecule to an aldehyde carbonyl group gives a hemiacetal.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Acetals are generally more stable than hemiacetals.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
Oxidation of hemiketal will give a ketone and an alcohol.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
Aldehydes are easier to oxidize than ketones.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
The simplest ketone has three carbon atoms.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


