Deck 16: Mixtures
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/141
Play
Full screen (f)
Deck 16: Mixtures
1
How would you classify the following material? coffee (black)
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound
C
2
Half-frozen fruit punch is always sweeter than the same fruit punch completely melted because
A)the sugar sinks to the bottom.
B)crystallization is a purifying process.
C)the half-frozen fruit punch is warmer.
D)sugar molecules are less soluble in a half-frozen solution.
A)the sugar sinks to the bottom.
B)crystallization is a purifying process.
C)the half-frozen fruit punch is warmer.
D)sugar molecules are less soluble in a half-frozen solution.
B
3
How would you classify the following material? milk
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound
A
4
What is the difference between a compound and a mixture?
A)A mixture can be physically separated into its components; a compound cannot be physically separated into its components.
B)A compound can be physically separated into its components; a mixture cannot be physically separated into its components.
C)A compound is just a mixture of elements.
D)The components of a mixture do not have the same properties individually as they do when mixed.
E)The components of a compound have the same properties individually as they do when mixed.
A)A mixture can be physically separated into its components; a compound cannot be physically separated into its components.
B)A compound can be physically separated into its components; a mixture cannot be physically separated into its components.
C)A compound is just a mixture of elements.
D)The components of a mixture do not have the same properties individually as they do when mixed.
E)The components of a compound have the same properties individually as they do when mixed.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a pure substance?
A)baking soda
B)salt water
C)cooking oil
D)duct tape
E)orange juice
A)baking soda
B)salt water
C)cooking oil
D)duct tape
E)orange juice

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following would be considered a homogeneous mixture?
A)wine
B)hydrogen cyanide
C)rusty iron
D)pretzel
E)sugar
A)wine
B)hydrogen cyanide
C)rusty iron
D)pretzel
E)sugar

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
7
Why can't the elements of a compound be separated from one another by physical means?
A)They are too homogeneous when found within a compound.
B)Their atoms are too tightly bound to one another.
C)Elements found within a compound tend to be inert.
D)Elements tend not to be soluble in water.
A)They are too homogeneous when found within a compound.
B)Their atoms are too tightly bound to one another.
C)Elements found within a compound tend to be inert.
D)Elements tend not to be soluble in water.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
8
When blue food coloring is stirred into water, the result is a ________.
A)homogeneous mixture called a solution
B)homogeneous mixture called a suspension
C)heterogeneous mixture called a solution
D)heterogeneous mixture called a suspension
E)pure liquid
A)homogeneous mixture called a solution
B)homogeneous mixture called a suspension
C)heterogeneous mixture called a solution
D)heterogeneous mixture called a suspension
E)pure liquid

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
9
Each circle represents an atom. Which of the following boxes contains an element? A compound? A mixture? 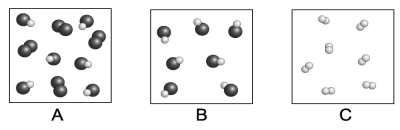
A)element: A, C; compound: A, B, C; mixture: A, B
B)element: C; compound: A, B; mixture: B
C)element: A, C; compound: A, B; mixture: A
D)element: A, C; compound: A, B; mixture: A, B

A)element: A, C; compound: A, B, C; mixture: A, B
B)element: C; compound: A, B; mixture: B
C)element: A, C; compound: A, B; mixture: A
D)element: A, C; compound: A, B; mixture: A, B

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
10
How would you classify the following material? swimming pool water
A)homogeneous mixture
B)heterogeneous mixture
C)a pure element
D)a pure compound
E)depends on how many children have been in it
A)homogeneous mixture
B)heterogeneous mixture
C)a pure element
D)a pure compound
E)depends on how many children have been in it

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
11
How might you separate a mixture of sand and salt?
A)with tweezers and a magnifying glass
B)just add water
C)heat the mixture until one of the components melts
D)Two of the above answers are reasonable.
A)with tweezers and a magnifying glass
B)just add water
C)heat the mixture until one of the components melts
D)Two of the above answers are reasonable.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
12
What is the difference between a compound and a mixture?
A)They both consist of atoms from different elements.
B)The way in which their atoms are bonded together.
C)One is a solid and the other is a liquid.
D)The components of a mixture are not chemically bonded together.
A)They both consist of atoms from different elements.
B)The way in which their atoms are bonded together.
C)One is a solid and the other is a liquid.
D)The components of a mixture are not chemically bonded together.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following would be considered a heterogeneous mixture?
A)salad dressing
B)water
C)milk
D)vegetable oil
E)vinegar
A)salad dressing
B)water
C)milk
D)vegetable oil
E)vinegar

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is a mixture?
A)air
B)gold
C)salt
D)iron
E)helium
A)air
B)gold
C)salt
D)iron
E)helium

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
15
Mixtures can be separated into their components by taking advantage of differences in the chemical properties of the components. Why might this separation method be less convenient than taking advantage of differences in the physical properties of the components?
A)A chemical property involves a chemical change so that you no longer have what you had.
B)Chemical properties are not as apparent as are physical properties.
C)The chemical properties of the components of a mixture are too similar to each other.
D)The chemical properties of the components of a mixture are too different from each other.
A)A chemical property involves a chemical change so that you no longer have what you had.
B)Chemical properties are not as apparent as are physical properties.
C)The chemical properties of the components of a mixture are too similar to each other.
D)The chemical properties of the components of a mixture are too different from each other.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
16
A combination of two or more substances in which they no longer retain their chemical properties is called a(n)________.
A)mixture
B)compound
C)heterogeneous mixture
D)periodic trend
E)suspension
A)mixture
B)compound
C)heterogeneous mixture
D)periodic trend
E)suspension

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
17
The following image represents which kind of matter? 
A)a mixture
B)a compound
C)an element
D)none of the above
E)all of the above

A)a mixture
B)a compound
C)an element
D)none of the above
E)all of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
18
Many dry cereals are fortified with iron, which is added to the cereal in the form of small iron particles. How might these particles be separated from the cereal?
A)add water and the iron particles will float to the top
B)blend the cereal to a fine consistency and pass through a filter
C)collect the iron filings with a magnet
D)heat the cereal so that the iron particles melt and thereby coalesce
A)add water and the iron particles will float to the top
B)blend the cereal to a fine consistency and pass through a filter
C)collect the iron filings with a magnet
D)heat the cereal so that the iron particles melt and thereby coalesce

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
19
If you filter sea water to remove all of the particles you would be left with a clear ________.
A)homogeneous mixture called a solution
B)homogeneous mixture called a suspension
C)heterogeneous mixture called a solution
D)heterogeneous mixture called a suspension
E)pure liquid
A)homogeneous mixture called a solution
B)homogeneous mixture called a suspension
C)heterogeneous mixture called a solution
D)heterogeneous mixture called a suspension
E)pure liquid

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
20
The following image represents which kind of matter? 
A)a compound
B)a mixture
C)an element
D)none of the above
E)all of the above

A)a compound
B)a mixture
C)an element
D)none of the above
E)all of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following boxes represents a suspension? 

 A B C
A B C
A)Only A represents a suspension.
B)Only B represents a suspension.
C)Only C represents a suspension.
D)Each of these could represent a submicroscopic portion of a suspension.


 A B C
A B CA)Only A represents a suspension.
B)Only B represents a suspension.
C)Only C represents a suspension.
D)Each of these could represent a submicroscopic portion of a suspension.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
22
In a solution made from one teaspoon of sugar and one liter of water, which is the solute?
A)sugar
B)water
C)the teaspoon
D)both sugar and water
E)none of the above
A)sugar
B)water
C)the teaspoon
D)both sugar and water
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
23
Is the air in your house a homogeneous or heterogeneous mixture?
A)homogeneous because it is mixed very well
B)heterogeneous because of the dust particles it contains
C)homogeneous because it is all at the same temperature
D)heterogeneous because it consists of different types of molecules
A)homogeneous because it is mixed very well
B)heterogeneous because of the dust particles it contains
C)homogeneous because it is all at the same temperature
D)heterogeneous because it consists of different types of molecules

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following describes the term concentration?
A)It is what you are doing now to answer this question.
B)It is the amount of solute in a given amount of solution.
C)It is the amount of solvent in a given amount of solution.
D)It is the given amount of solution in a given container.
E)It is the given amount of solvent per amount of solute.
A)It is what you are doing now to answer this question.
B)It is the amount of solute in a given amount of solution.
C)It is the amount of solvent in a given amount of solution.
D)It is the given amount of solution in a given container.
E)It is the given amount of solvent per amount of solute.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
25
Classify the following as element, compound, or mixture, and justify your classifications: table salt, stainless steel, table sugar, aluminum, ice.
A)mixture; element; compound; element; element
B)compound; mixture; compound element; compound
C)mixture; compound; mixture; element; compound
D)compound; element; compound; element; compound
A)mixture; element; compound; element; element
B)compound; mixture; compound element; compound
C)mixture; compound; mixture; element; compound
D)compound; element; compound; element; compound

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
26
What can be said about drinking water that is 99.9999 percent free of some poison, such as a pesticide?
A)In each 10,000 parts of the contaminated water there is one part pesticide and 9999 parts pure water.
B)In each 100,000 parts of the contaminated water there is one part pesticide and 99,999 parts pure water.
C)The ratio of water molecules to pesticide molecules in the glass is so great that drinking the water is not problematic.
D)The water is highly contaminated and surely not fit to drink.
A)In each 10,000 parts of the contaminated water there is one part pesticide and 9999 parts pure water.
B)In each 100,000 parts of the contaminated water there is one part pesticide and 99,999 parts pure water.
C)The ratio of water molecules to pesticide molecules in the glass is so great that drinking the water is not problematic.
D)The water is highly contaminated and surely not fit to drink.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following solutions is the most dilute?
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following solutions is the most concentrated?
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
29
How would you classify the following material? coffee (with milk)
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
30
A sample of water that is 99.9999 percent pure contains 0.0001 percent impurities. Consider from Chapter 1 that a glass of water contains on the order of a trillion trillion (1 × 1024)molecules. If 0.0001 percent of these molecules were the molecules of some impurity, about how many impurity molecules would this be?
A)1000 (one thousand: 1 × 103)
B)1,000,000 (one million: 1 × 106)
C)1,000,000,000 (one billion: 1 × 109)
D)1,000,000,000,000,000,000 (one million trillion: 1 × 1018)
A)1000 (one thousand: 1 × 103)
B)1,000,000 (one million: 1 × 106)
C)1,000,000,000 (one billion: 1 × 109)
D)1,000,000,000,000,000,000 (one million trillion: 1 × 1018)

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
31
What do chicken noodle soup and garden soil have in common?
A)They are both examples of heterogeneous mixtures.
B)They both contain elements.
C)They are both examples of compounds.
D)nothing
A)They are both examples of heterogeneous mixtures.
B)They both contain elements.
C)They are both examples of compounds.
D)nothing

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
32
In a solution of 77 percent nitrogen and 23 percent oxygen, which is the solvent?
A)nitrogen
B)oxygen
C)both
D)neither
E)Gases cannot form solutions.
A)nitrogen
B)oxygen
C)both
D)neither
E)Gases cannot form solutions.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following solutions is the most dilute?
A)0.1 liter of water with 1 gram of sugar
B)0.2 liter of water with 2 grams of sugar
C)0.5 liter of water with 5 grams of sugar
D)1 liter of water with 10 grams of sugar
E)They all have the same concentration.
A)0.1 liter of water with 1 gram of sugar
B)0.2 liter of water with 2 grams of sugar
C)0.5 liter of water with 5 grams of sugar
D)1 liter of water with 10 grams of sugar
E)They all have the same concentration.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
34
Read carefully. Twice as much as one million trillion is two million trillion. One thousand times as much is 1000 million trillion. One million times as much is 1,000,000 million trillion, which is the same as one trillion trillion. Thus, one trillion trillion is a million times greater than a million trillion. Got that? So how many more water molecules than impurity molecules are there in a glass of water that is 99.9999 percent pure?
A)1000 (one thousand: 1 × 103)more water molecules than impurities molecules
B)1,000,000 (one million: 1 × 106)more water molecules than impurities molecules
C)1,000,000,000 (one billion: 1 × 109)more water molecules than impurities molecules
D)1,000,000,000,000,000,000 (one million trillion: 1 × 1018)more water molecules than impurities molecules
A)1000 (one thousand: 1 × 103)more water molecules than impurities molecules
B)1,000,000 (one million: 1 × 106)more water molecules than impurities molecules
C)1,000,000,000 (one billion: 1 × 109)more water molecules than impurities molecules
D)1,000,000,000,000,000,000 (one million trillion: 1 × 1018)more water molecules than impurities molecules

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements describes a saturated solution?
A)a solution where the solvent cannot dissolve any more solute
B)a solution of salt water with salt at the bottom
C)a carbonated beverage with bubbles
D)all of the above
E)none of the above
A)a solution where the solvent cannot dissolve any more solute
B)a solution of salt water with salt at the bottom
C)a carbonated beverage with bubbles
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
36
Someone argues that he or she doesn't drink tap water because it contains thousands of molecules of some impurity in each glass. How would you respond in defense of the water's purity, if it indeed does contain thousands of molecules of some impurity per glass?
A)Impurities aren't necessarily bad, in fact, they may be good for you.
B)The water contains water molecules and each water molecule is pure.
C)There's no defense. If the water contains impurities it should not be drunk.
D)Compared to the billions and billions of water molecules, a thousand molecules of something else is practically nothing.
A)Impurities aren't necessarily bad, in fact, they may be good for you.
B)The water contains water molecules and each water molecule is pure.
C)There's no defense. If the water contains impurities it should not be drunk.
D)Compared to the billions and billions of water molecules, a thousand molecules of something else is practically nothing.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following material phases cannot form a solution?
A)solids
B)liquids
C)gases
D)All of the above can form solutions.
E)None of the above can form solutions.
A)solids
B)liquids
C)gases
D)All of the above can form solutions.
E)None of the above can form solutions.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
38
How would you classify the following material? a cappuccino (with foam)
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound
A)a suspension
B)a heterogeneous mixture
C)a solution
D)an element
E)a compound

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
39
How does a suspension differ from a solution?
A)A suspension is a heterogeneous mixture whose components can be separated by simple filtration. A solution is a homogeneous mixture which cannot be separated by simple filtration.
B)A suspension is a heterogeneous mixture consisting of different phases whereas a solution is a homogeneous mixture consisting of a single phase.
C)Although a solution and suspension are both homogeneous mixtures, only the components of a suspension will separate by spinning the mixture in a centrifuge.
D)The difference between a suspension and a solution can only be determined by chemical means.
A)A suspension is a heterogeneous mixture whose components can be separated by simple filtration. A solution is a homogeneous mixture which cannot be separated by simple filtration.
B)A suspension is a heterogeneous mixture consisting of different phases whereas a solution is a homogeneous mixture consisting of a single phase.
C)Although a solution and suspension are both homogeneous mixtures, only the components of a suspension will separate by spinning the mixture in a centrifuge.
D)The difference between a suspension and a solution can only be determined by chemical means.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of steel is composed of 5 percent carbon and 95 percent iron. Which is the solvent?
A)iron
B)carbon
C)steel
D)Steel is not a solution, it is a mixture.
E)A solid cannot be a solvent.
A)iron
B)carbon
C)steel
D)Steel is not a solution, it is a mixture.
E)A solid cannot be a solvent.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
41
How many grams of sugar (sucrose)are there in 5 liters of sugar water that has a concentration of 0.5 grams per liter of solution?
A)50 g
B)25 g
C)2.5 g
D)1.5 g
A)50 g
B)25 g
C)2.5 g
D)1.5 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
42
How many molecules of sucrose are in 0.5 L of a 2 molar solution of sucrose?
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following best describes a two-molar sucrose solution?
A)one liter of solution that contains 2 moles of sucrose
B)one liter of solution that contains 2 moles of water
C)one liter of solution that contains 6.02 × 1023 molecules of sucrose
D)two liters of solution that contains 1 mole of sucrose
E)one mole of sucrose dissolved in 2 liters of solution
A)one liter of solution that contains 2 moles of sucrose
B)one liter of solution that contains 2 moles of water
C)one liter of solution that contains 6.02 × 1023 molecules of sucrose
D)two liters of solution that contains 1 mole of sucrose
E)one mole of sucrose dissolved in 2 liters of solution

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
44
If you need 3.01 × 1023 molecules of sucrose, how many liters of a 4.00 molar solution would you need?
A)0.125 L
B)0.250 L
C)4.00 L
D)1.00 L
E)none of the above
A)0.125 L
B)0.250 L
C)4.00 L
D)1.00 L
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
45
What is the molarity when water is added to 2 moles of sodium chloride to make 0.5 liter of solution?
A)8 M
B)4 M
C)5 M
D)2.5 M
A)8 M
B)4 M
C)5 M
D)2.5 M

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
46
If you need 10 moles of sucrose, how many liters of a 4.0 molar solution would you need?
A)2.5 L
B)0.25 L
C)25 L
D)10. L
E)none of the above
A)2.5 L
B)0.25 L
C)25 L
D)10. L
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
47
How many moles of sugar, C12H22O11, are there in 200. grams?
A)0.585 moles
B)68,400 moles
C)1.71 moles
D)0.684 moles
A)0.585 moles
B)68,400 moles
C)1.71 moles
D)0.684 moles

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
48
What is the sum of the atomic masses of all the atoms in sucrose, C12H22O11?
A)342 amu
B)182 amu
C)270 amu
D)none of the above
A)342 amu
B)182 amu
C)270 amu
D)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
49
How many grams of sodium chloride are needed to make 15 L of a solution that has a concentration of 3.0 g per liter of solution?
A)30 g
B)141 g
C)5 g
D)45 g
A)30 g
B)141 g
C)5 g
D)45 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
50
Which has the most atoms?
A)a mole of gold
B)a mole of helium
C)a mole of lead
D)All of the above have the same number of atoms.
E)none of the above
A)a mole of gold
B)a mole of helium
C)a mole of lead
D)All of the above have the same number of atoms.
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
51
What statement best describes a mole?
A)a little furry mammal that lives in the ground
B)a very small number chemists use to count atoms or molecules
C)the amount of molecules or atoms in 1 gram of something
D)It is a very large number chemists use to count atoms or molecules.
E)none of the above
A)a little furry mammal that lives in the ground
B)a very small number chemists use to count atoms or molecules
C)the amount of molecules or atoms in 1 gram of something
D)It is a very large number chemists use to count atoms or molecules.
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following solutions is the most concentrated?
A)0.5 L of a 3 molar solution
B)3.0 L of a 0.5 molar solution
C)2.0 L of a 1 molar solution
D)0.5 L of a 1 molar solution
E)2.0 L of a 2 molar solution
A)0.5 L of a 3 molar solution
B)3.0 L of a 0.5 molar solution
C)2.0 L of a 1 molar solution
D)0.5 L of a 1 molar solution
E)2.0 L of a 2 molar solution

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
53
What is molarity?
A)the number of moles of solute per liter of solution
B)the number of grams of solute per liter of solution
C)the number of moles of solute per liter of solvent
D)the number of liters of solute per mole of solution
E)none of the above
A)the number of moles of solute per liter of solution
B)the number of grams of solute per liter of solution
C)the number of moles of solute per liter of solvent
D)the number of liters of solute per mole of solution
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
54
How many moles of water are there in 100. grams of water?
A)1800 moles
B)100 moles
C)0.018 moles
D)5.55 moles
A)1800 moles
B)100 moles
C)0.018 moles
D)5.55 moles

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
55
Many solvents expand to occupy greater volumes with increasing temperature. What happens to the concentration of a solution made with such a solvent as its temperature is increased?
A)Since concentration depends on how much mass is dissolved in a given volume, as the volume increases, the concentration decreases.
B)The concentration of a solution increases as the solute fits into the new spaces between the molecules.
C)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has decreased.
D)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has increased.
A)Since concentration depends on how much mass is dissolved in a given volume, as the volume increases, the concentration decreases.
B)The concentration of a solution increases as the solute fits into the new spaces between the molecules.
C)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has decreased.
D)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has increased.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
56
A student is told to use 20.0 grams of sodium chloride to make an aqueous solution that has a concentration of 10.0 grams of sodium chloride per liter of solution. Assuming that 20.0 grams of sodium chloride has a volume of 7.5 milliliters, about how much water will she use in making this solution?
A)9.25 L
B)9.5 L
C)9.9925 L
D)10 L
A)9.25 L
B)9.5 L
C)9.9925 L
D)10 L

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
57
How many molecules of sucrose are in a 0.5 moles of sucrose?
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
58
What is the molarity of 0.5 liters of a solution with five moles of sucrose in it?
A)10 molar
B)0.5 molar
C)5 molar
D)2.5 molar
E)1 molar
A)10 molar
B)0.5 molar
C)5 molar
D)2.5 molar
E)1 molar

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
59
How many molecules of sucrose are in 0.5 L of a 1 molar solution of sucrose?
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following solutions is the most concentrated?
A)0.1 liter of water with 1 gram of sugar
B)2 liters of water with 0.2 gram of sugar
C)0.5 liter of water with 50 grams of sugar
D)3 liters of water with 30 grams of sugar
E)They all have the same concentration.
A)0.1 liter of water with 1 gram of sugar
B)2 liters of water with 0.2 gram of sugar
C)0.5 liter of water with 50 grams of sugar
D)3 liters of water with 30 grams of sugar
E)They all have the same concentration.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
61
Why are noble gases infinitely soluble in noble gases?
A)The noble gases are attracted to each other by induced dipole-induced dipole attractions, but there is only one attraction per molecule.
B)These are the smallest atoms on the periodic table.
C)The molecules do not interact with each other, excluding other molecules.
D)Noble gases can be mixed homogeneously in any proportion.
A)The noble gases are attracted to each other by induced dipole-induced dipole attractions, but there is only one attraction per molecule.
B)These are the smallest atoms on the periodic table.
C)The molecules do not interact with each other, excluding other molecules.
D)Noble gases can be mixed homogeneously in any proportion.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
62
Why does oxygen have such a low solubility in water?
A)Water's attraction for itself is stronger than its attraction for oxygen molecules.
B)Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C)The hydrogen bonding in water keeps the oxygen solubility low.
D)Both A and B are true.
A)Water's attraction for itself is stronger than its attraction for oxygen molecules.
B)Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C)The hydrogen bonding in water keeps the oxygen solubility low.
D)Both A and B are true.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following might have the best solubility in water?
A)CH3OH
B)Cl2
C)O2
D)CH3CH3
E)none of the above
A)CH3OH
B)Cl2
C)O2
D)CH3CH3
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
64
Based on atomic size, which would you expect to be more soluble in water: helium, He, or nitrogen, N2?
A)Although He is smaller, its outer orbital is filled and the atom will have little attraction to the water molecules.
B)Since He atoms are smaller, more of them can fit into solution, so it has a higher solubility in water.
C)Nitrogen atoms are bigger and so nitrogen molecules should be more soluble in water due to greater dipole-induced dipole attractions.
D)He atoms are bigger and so helium molecules should be more soluble in water due to greater dipole-induced dipole attractions.
A)Although He is smaller, its outer orbital is filled and the atom will have little attraction to the water molecules.
B)Since He atoms are smaller, more of them can fit into solution, so it has a higher solubility in water.
C)Nitrogen atoms are bigger and so nitrogen molecules should be more soluble in water due to greater dipole-induced dipole attractions.
D)He atoms are bigger and so helium molecules should be more soluble in water due to greater dipole-induced dipole attractions.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
65
Why does oxygen have such a low solubility in water?
A)Water's attraction for itself is stronger than its attraction for oxygen molecules.
B)Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C)The hydrogen bonding in water keeps the oxygen solubility low.
D)Both A and B are true.
A)Water's attraction for itself is stronger than its attraction for oxygen molecules.
B)Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C)The hydrogen bonding in water keeps the oxygen solubility low.
D)Both A and B are true.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
66
How can you tell whether a sugar solution is saturated or not?
A)Add more sugar, if it dissolves, it is saturated.
B)There will be a precipitate if the water is heated.
C)Cool the solution to see if there is a precipitate.
D)As long as there are more water molecules than sugar molecules, there is a saturated solution.
A)Add more sugar, if it dissolves, it is saturated.
B)There will be a precipitate if the water is heated.
C)Cool the solution to see if there is a precipitate.
D)As long as there are more water molecules than sugar molecules, there is a saturated solution.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
67
What happens when the molecule-to-molecule attractions in the solute are comparable to those in the solvent?
A)The solute can have infinite solubility in the solvent.
B)The solute does not dissolve in the solvent.
C)The material has only limited solubility in the solvent.
D)The solution will become saturated.
E)none of the above
A)The solute can have infinite solubility in the solvent.
B)The solute does not dissolve in the solvent.
C)The material has only limited solubility in the solvent.
D)The solution will become saturated.
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
68
How is the solubility of a gas affected by temperature?
A)As temperature goes up, the solubility goes up.
B)As temperature goes down, the solubility goes down.
C)As temperature goes up, the solubility stays the same.
D)As temperature goes down, the solubility goes up.
E)both A and B
A)As temperature goes up, the solubility goes up.
B)As temperature goes down, the solubility goes down.
C)As temperature goes up, the solubility stays the same.
D)As temperature goes down, the solubility goes up.
E)both A and B

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following might have the lowest solubility in water?
A)CH3OH
B)Cl2
C)O2
D)CH3CH3
E)none of the above
A)CH3OH
B)Cl2
C)O2
D)CH3CH3
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
70
Describe what usually happens to a hot solution that is saturated with a solid as it cools.
A)The solid that is dissolved comes out of the solution completely.
B)The solid stays in the solution.
C)Some of the solid comes out of the solution.
D)The solution freezes.
E)The solution solidifies.
A)The solid that is dissolved comes out of the solution completely.
B)The solid stays in the solution.
C)Some of the solid comes out of the solution.
D)The solution freezes.
E)The solution solidifies.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
71
How are intermolecular forces and solubility related?
A)Solubility depends on the solvent's ability to overcome the intermolecular forces in a solid.
B)Solubility depends on the solute's ability to overcome the intermolecular forces in the solvent.
C)Solubility is a measure of how strong a solvent's intermolecular forces are.
D)Solubility is a measure of how weak the intermolecular forces in the solute are.
E)none of the above
A)Solubility depends on the solvent's ability to overcome the intermolecular forces in a solid.
B)Solubility depends on the solute's ability to overcome the intermolecular forces in the solvent.
C)Solubility is a measure of how strong a solvent's intermolecular forces are.
D)Solubility is a measure of how weak the intermolecular forces in the solute are.
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
72
If you were to increase the pressure of a gas above a liquid (such as by pressing a piston above a liquid)what happens?
A)The gas is forced into solution and the solubility increases.
B)The solution is compressed and the gas is forced out of the solvent.
C)The pressure goes down and the gas moves out of the solvent.
D)The pressure goes down and the gas goes into the solvent.
E)The amount of gas in the solution would stay the same.
A)The gas is forced into solution and the solubility increases.
B)The solution is compressed and the gas is forced out of the solvent.
C)The pressure goes down and the gas moves out of the solvent.
D)The pressure goes down and the gas goes into the solvent.
E)The amount of gas in the solution would stay the same.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
73
If the solubility of a compound is 72 grams per liter, how many grams of the compound will dissolve in 0.50 liters?
A)36 g
B)72 g
C)144 g
D)30 g
E)none of the above
A)36 g
B)72 g
C)144 g
D)30 g
E)none of the above

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
74
If the solubility of a compound is 30 grams per liter, how much solid is left undissolved if you mix 30 g of the compound in 0.33 L of solution?
A)20 g
B)10 g
C)0 g
D)30 g
E)33 g
A)20 g
B)10 g
C)0 g
D)30 g
E)33 g

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
75
If nitrogen, N2, were pumped into your lungs at high pressure, what would happen to its solubility in your blood stream?
A)The greater the pressure, the greater the solubility.
B)The greater the pressure, the lower the solubility.
C)You cannot change solubility of a substance by changing the pressure.
D)Nitrogen is not soluble in your blood.
A)The greater the pressure, the greater the solubility.
B)The greater the pressure, the lower the solubility.
C)You cannot change solubility of a substance by changing the pressure.
D)Nitrogen is not soluble in your blood.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
76
A solid has a solubility at room temperature of 78 grams per liter. If 1.0 L of a hot solution containing 100. g of solute is cooled to room temperature, how much solid is formed?
A)22 g
B)100 g
C)78 g
D)1 L
E)78g/L
A)22 g
B)100 g
C)78 g
D)1 L
E)78g/L

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
77
Under which of the following conditions would you expect the highest solubility of oxygen gas in water?
A)high temperature and low O2 pressure above the solution
B)low temperature and high O2 pressure above the solution
C)low temperature and low O2 pressure above the solution
D)high temperature and high O2 pressure above the solution
E)The O2 solubility is independent of temperature and pressure.
A)high temperature and low O2 pressure above the solution
B)low temperature and high O2 pressure above the solution
C)low temperature and low O2 pressure above the solution
D)high temperature and high O2 pressure above the solution
E)The O2 solubility is independent of temperature and pressure.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
78
The air that a scuba diver breathes is pressurized to counteract the pressure exerted by the surrounding water. Under these conditions, excessive amounts of nitrogen dissolves in bodily fluids, such as blood. If the diver ascends to the surface too rapidly, the excessive nitrogen bubbles out of the bodily fluids-much like carbon dioxide bubbles out of a soda immediately after its has been opened. This results in a painful and potentially lethal medical condition known as the bends. Why does breathing a mixture of helium and oxygen rather than air help divers to avoid getting the bends?
A)Oxygen and helium have stronger attractions for each other than they do for the blood, so less helium will be dissolved in the blood and to cause the bends.
B)The helium is less soluble in the bodily fluids and so less dissolves for a given pressure. Upon decompression, there is less helium to "bubble out" and cause potential harm.
C)The nitrogen in the blood will bind to helium, and so will be exhaled rather than being stuck in the blood.
D)Helium is a smaller molecule than nitrogen, so when it bubbles out of solution, it is less painful and less harmful to the body.
A)Oxygen and helium have stronger attractions for each other than they do for the blood, so less helium will be dissolved in the blood and to cause the bends.
B)The helium is less soluble in the bodily fluids and so less dissolves for a given pressure. Upon decompression, there is less helium to "bubble out" and cause potential harm.
C)The nitrogen in the blood will bind to helium, and so will be exhaled rather than being stuck in the blood.
D)Helium is a smaller molecule than nitrogen, so when it bubbles out of solution, it is less painful and less harmful to the body.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
79
What property primarily determines the effect of temperature on the solubility of gas molecules?
A)the kinetic energy of the gas
B)the polarity of the gas
C)the molecular weight of the gas
D)the ionic strength of the gas
E)the dipole strength of the solvent
A)the kinetic energy of the gas
B)the polarity of the gas
C)the molecular weight of the gas
D)the ionic strength of the gas
E)the dipole strength of the solvent

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
80
How is the solubility of a solid affected by temperature?
A)As temperature goes up, the solubility goes up.
B)As temperature goes down, the solubility goes down.
C)As temperature goes up, the solubility goes down.
D)As temperature goes down, the solubility goes up.
E)both A and B
A)As temperature goes up, the solubility goes up.
B)As temperature goes down, the solubility goes down.
C)As temperature goes up, the solubility goes down.
D)As temperature goes down, the solubility goes up.
E)both A and B

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck


