Deck 18: Two Classes of Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/182
Play
Full screen (f)
Deck 18: Two Classes of Chemical Reactions
1
According to the following reaction, which molecule is acting as a base? H2O + H2SO4 → H3O+ + HSO4-
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
B
2
According to the following reaction, which molecule is acting as a base? H2O + NH3 → OH- + NH4+
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
B
3
According to the following reaction, which molecule is acting as an acid? OH- + NH4+ → H2O + NH3
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
D
4
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
5
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
6
In the reaction below, what does the symbol ⇌ mean? OH- + NH4+ ⇌ H2O + NH3
A)It means that the forward and backward reactions are happening at the same time.
B)It means that the reaction cannot decide which way to go.
C)It means that the forward reaction does not progress.
D)It means that the backward reaction happens as fast as the forward reaction and so the proton is not transferred at all.
E)none of the above
A)It means that the forward and backward reactions are happening at the same time.
B)It means that the reaction cannot decide which way to go.
C)It means that the forward reaction does not progress.
D)It means that the backward reaction happens as fast as the forward reaction and so the proton is not transferred at all.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds would least likely act as an acid?
A)SO4-2
B)HSO4-1
C)H2SO4
D)NH3
E)CH3CO2H
A)SO4-2
B)HSO4-1
C)H2SO4
D)NH3
E)CH3CO2H

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
8
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
9
According to the following reaction, which molecule is acting as an acid? H2O + NH3 → OH- + NH4+
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
10
For the following acid-base reaction, identify what compound is formed in the space marked. HCl + KOH ⇌ ???? + H2O
A)KCl
B)H3OCl
C)KOH2
D)KOH2Cl
E)none of the above
A)KCl
B)H3OCl
C)KOH2
D)KOH2Cl
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
11
According to the following reaction, which molecule is acting as a base? OH- + NH4+ → H2O + NH3
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above
A)H2O
B)NH3
C)OH-
D)NH4+
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
12
According to the following reaction, which molecule is acting as an acid? H2O + H2SO4 → H3O+ + HSO4-
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
13
How do you make a proton out of a hydrogen atom?
A)remove an electron from a hydrogen atom
B)remove a proton from a helium nucleus
C)let the hydrogen atoms undergo fusion
D)let the hydrogen atoms combine to form a hydrogen molecule and eject an electron
E)let the hydrogen atoms combine to form a hydrogen molecule and eject a proton
A)remove an electron from a hydrogen atom
B)remove a proton from a helium nucleus
C)let the hydrogen atoms undergo fusion
D)let the hydrogen atoms combine to form a hydrogen molecule and eject an electron
E)let the hydrogen atoms combine to form a hydrogen molecule and eject a proton

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
14
What is a base?
A)Anything that accepts a hydrogen ion.
B)Anything that accepts a hydroxide ion.
C)Anything that donates a hydroxide ion.
D)Anything that can be used to clean drains.
E)Anything with a bitter taste.
A)Anything that accepts a hydrogen ion.
B)Anything that accepts a hydroxide ion.
C)Anything that donates a hydroxide ion.
D)Anything that can be used to clean drains.
E)Anything with a bitter taste.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
15
What best describes what happens when an acid such as HCl is mixed with water?
A)The proton chemically bonded to the chlorine is transferred to a water molecule and forms a chloride ion and a hydronium ion.
B)A proton from the chlorine nucleus is ejected and captured by a water molecule to form a negatively charged HCl and a new hydronium ion.
C)A hydroxide ion from the water is transferred to the HCl molecule to form a proton and hydronium ion.
D)HCl is not an acid.
E)none of the above
A)The proton chemically bonded to the chlorine is transferred to a water molecule and forms a chloride ion and a hydronium ion.
B)A proton from the chlorine nucleus is ejected and captured by a water molecule to form a negatively charged HCl and a new hydronium ion.
C)A hydroxide ion from the water is transferred to the HCl molecule to form a proton and hydronium ion.
D)HCl is not an acid.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
16
What is an acid?
A)Anything that donates hydrogen ions.
B)Anything that accepts hydrogen atoms.
C)Anything that donates hydrogen atoms.
D)Anything that dissolves metal.
E)Anything that donates hydronium ions.
A)Anything that donates hydrogen ions.
B)Anything that accepts hydrogen atoms.
C)Anything that donates hydrogen atoms.
D)Anything that dissolves metal.
E)Anything that donates hydronium ions.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
17
According to the following reaction, which molecule is acting as an acid? H3O+ + HSO4- → H2O + H2SO4
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
18
For the following acid-base reaction, identify which salt is formed. HCl + NaOH ⇌ ???? + H2O
A)NaCl
B)H3OCl
C)NaOH2
D)NaOH2Cl
E)none of the above
A)NaCl
B)H3OCl
C)NaOH2
D)NaOH2Cl
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
19
For the following reaction, identify whether the compound in bold is behaving as an acid or a base. H3PO4 + H2O ⇌ H2PO4- + H3O+
A)acid
B)base
C)neither
D)both
E)none of the above
A)acid
B)base
C)neither
D)both
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
20
According to the following reaction, which molecule is acting as a base? H3O+ + HSO4- → H2O + H2SO4
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above
A)H2SO4
B)H2O
C)H3O+
D)HSO4-
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
21
What is the main characteristic of a strong base?
A)It is completely dissociated in water.
B)It readily accepts an acidic proton.
C)It is corrosive.
D)It will damage your skin.
E)all of the above
A)It is completely dissociated in water.
B)It readily accepts an acidic proton.
C)It is corrosive.
D)It will damage your skin.
E)all of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
22
What happens to the corrosive properties of an acid and a base after they neutralize each other? Why?
A)The corrosive properties are neutralized because the acid and base no longer exist.
B)The corrosive properties are unaffected because salt is a corrosive agent.
C)The corrosive properties are doubled because the acid and base are combined in the salt.
D)The corrosive properties remain the same when the salt is mixed into water.
A)The corrosive properties are neutralized because the acid and base no longer exist.
B)The corrosive properties are unaffected because salt is a corrosive agent.
C)The corrosive properties are doubled because the acid and base are combined in the salt.
D)The corrosive properties remain the same when the salt is mixed into water.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
23
Identify the acid or base behavior of each substance in these reactions: H3O+ + Cl- ⇌ H2O + HCl
A)H3O+ acts as an acid, Cl- acts as a base, H2O acts an acid, HCl acts as a base.
B)H3O+ acts as an base, Cl- acts as a acid, H2O acts an acid, HCl acts as a base.
C)H3O+ acts as an acid, Cl- acts as a base, H2O acts an base, HCl acts as a acid.
D)H3O+ acts as an base, Cl-
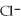 acts as a acid, H2O acts an base, HCl acts as an acid.
acts as a acid, H2O acts an base, HCl acts as an acid.
A)H3O+ acts as an acid, Cl- acts as a base, H2O acts an acid, HCl acts as a base.
B)H3O+ acts as an base, Cl- acts as a acid, H2O acts an acid, HCl acts as a base.
C)H3O+ acts as an acid, Cl- acts as a base, H2O acts an base, HCl acts as a acid.
D)H3O+ acts as an base, Cl-
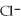 acts as a acid, H2O acts an base, HCl acts as an acid.
acts as a acid, H2O acts an base, HCl acts as an acid.
Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
24
The main component of bleach is sodium hypochlorite, NaOCl, which consists of sodium ions, Na⁺, and hypochlorite ions, -OCl What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
A)The products are NaCl, O2, and HClO2.
B)The products are NaOH, H2O, and Cl2
C)The products are NaCl and HOCl.
D)The products are NaOH, O2, and Cl2
A)The products are NaCl, O2, and HClO2.
B)The products are NaOH, H2O, and Cl2
C)The products are NaCl and HOCl.
D)The products are NaOH, O2, and Cl2

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements about strong and weak acids is not true?
A)A weak acid is as corrosive as a strong acid.
B)A weak acid dissociates in water.
C)A strong acid will react with a strong base.
D)A weak acid will react with a strong base.
E)All of the above are untrue.
A)A weak acid is as corrosive as a strong acid.
B)A weak acid dissociates in water.
C)A strong acid will react with a strong base.
D)A weak acid will react with a strong base.
E)All of the above are untrue.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
26
Water is formed from the reaction of an acid and a base. Why is it not classified as a salt?
A)Not all acid base reactions produce a salt, as in the case with the formation of water.
B)The attraction between the two ions in water molecules are too strong.
C)By definition, a salt must be able to dissolve in water, so water itself cannot be called a salt.
D)A salt is an ionic compound, whereas water is a covalent compound.
A)Not all acid base reactions produce a salt, as in the case with the formation of water.
B)The attraction between the two ions in water molecules are too strong.
C)By definition, a salt must be able to dissolve in water, so water itself cannot be called a salt.
D)A salt is an ionic compound, whereas water is a covalent compound.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
27
Identify the acid or base behavior of each substance in these reactions: HSO4- + H2O ⇌ OH- + H2S 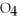
A)HS O4- acts as an acid, H2O acts as a base, OH- acts as an acid, H2S O4 acts as a base.
B)HS O4- acts as an base, H2O acts as a acid, OH- acts as an acid, H2S O4 acts as a base.
C)HS O4- acts as an acid, H2O acts as a base, OH- acts as an base, H2S O4 acts as a acid.
D)HS O4- acts as an base, H2O acts as a acid, OH- acts as an base, H2S O4 acts as a acid.
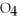
A)HS O4- acts as an acid, H2O acts as a base, OH- acts as an acid, H2S O4 acts as a base.
B)HS O4- acts as an base, H2O acts as a acid, OH- acts as an acid, H2S O4 acts as a base.
C)HS O4- acts as an acid, H2O acts as a base, OH- acts as an base, H2S O4 acts as a acid.
D)HS O4- acts as an base, H2O acts as a acid, OH- acts as an base, H2S O4 acts as a acid.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
28
An acid and a base react to form a salt, which consists of positive and negative ions. Which forms the positive ions: the acid or the base? Which forms the negative ions?
A)The acid forms the positive ion, the base forms the negative ion.
B)The acid forms the negative ion, the base forms the positive ion.
C)Because different substances can act as an acid or a base, it depends on the substance you begin with.
D)all of the above
A)The acid forms the positive ion, the base forms the negative ion.
B)The acid forms the negative ion, the base forms the positive ion.
C)Because different substances can act as an acid or a base, it depends on the substance you begin with.
D)all of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the above images would best describe a water solution of a weak acid (HA)the best?
A)A
B)B
C)C
D)All of the above are weak acids.
E)None of the above are weak acids.
A)A
B)B
C)C
D)All of the above are weak acids.
E)None of the above are weak acids.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
30
What is the main characteristic of a strong acid?
A)It is completely dissociated in water.
B)It readily gives up its proton to a base.
C)It is corrosive.
D)It will damage your skin.
E)all of the above
A)It is completely dissociated in water.
B)It readily gives up its proton to a base.
C)It is corrosive.
D)It will damage your skin.
E)all of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
31
For the following acid-base reaction, identify what is formed in the space marked. HF + KOH ⇌ ???? + H2O
A)KF
B)H3OF
C)KOH2
D)KOH2F
E)none of the above
A)KF
B)H3OF
C)KOH2
D)KOH2F
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements is not true about a neutralization reaction?
A)Water is always formed in a neutralization reaction.
B)One molecule of acid neutralizes one molecule of base.
C)A neutralization is the reaction of a hydroxide ion with a proton.
D)All of the above are true.
E)None of the above are true.
A)Water is always formed in a neutralization reaction.
B)One molecule of acid neutralizes one molecule of base.
C)A neutralization is the reaction of a hydroxide ion with a proton.
D)All of the above are true.
E)None of the above are true.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
33
Suggest why people once washed their hands with ashes.
A)The ashes act as a base and reacts with skin oils to produce solutions of soap.
B)The ashes act as an acid and reacts with the skin oils to produce soap.
C)The oils on the skin act as a base and reacts with the ashes to produce soap.
D)After being burnt in the fire, the acids and bases of the log are neutralized, making it a gentle material to use on the hands.
A)The ashes act as a base and reacts with skin oils to produce solutions of soap.
B)The ashes act as an acid and reacts with the skin oils to produce soap.
C)The oils on the skin act as a base and reacts with the ashes to produce soap.
D)After being burnt in the fire, the acids and bases of the log are neutralized, making it a gentle material to use on the hands.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
34
What is the relationship between the hydroxide ion and a water molecule?
A)A hydroxide ion is a water molecule plus a proton.
B)A hydroxide ion and a water molecule are the same things.
C)A hydroxide ion is a water molecule minus a hydrogen nucleus.
D)A hydroxide ion is a water molecule plus two extra electrons.
A)A hydroxide ion is a water molecule plus a proton.
B)A hydroxide ion and a water molecule are the same things.
C)A hydroxide ion is a water molecule minus a hydrogen nucleus.
D)A hydroxide ion is a water molecule plus two extra electrons.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the above images represents a solution of the strongest acid (HA)?
A)A
B)B
C)C
D)All of the above are strong acids.
E)None of the above are strong acids.
A)A
B)B
C)C
D)All of the above are strong acids.
E)None of the above are strong acids.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements about strong or weak acids is true?
A)A weak acid and a strong acid at the same concentration are equally corrosive.
B)A weak acid readily forms ions when dissolved in water.
C)A strong acid will never react with a strong base.
D)A weak acid will react with a strong base.
A)A weak acid and a strong acid at the same concentration are equally corrosive.
B)A weak acid readily forms ions when dissolved in water.
C)A strong acid will never react with a strong base.
D)A weak acid will react with a strong base.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements about strong or weak bases is true?
A)A weak base completely separates into ions in water.
B)A weak base will not react with a strong acid.
C)A strong base is always corrosive.
D)A strong base will readily accept protons from even weak acids.
A)A weak base completely separates into ions in water.
B)A weak base will not react with a strong acid.
C)A strong base is always corrosive.
D)A strong base will readily accept protons from even weak acids.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
38
For the following acid-base reaction, identify what is formed in the space marked. H2SO4 + KOH ⇌ KHSO4 + ???
A)K2SO4
B)H3OSO4
C)H2O
D)KOH2SO4
E)none of the above
A)K2SO4
B)H3OSO4
C)H2O
D)KOH2SO4
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements about strong and weak bases is not true?
A)A weak base does not completely dissociate in water.
B)A weak base will not react with a strong acid.
C)A strong base can be extremely corrosive.
D)A strong base will readily accept protons from even weak acids.
E)All of the above are untrue.
A)A weak base does not completely dissociate in water.
B)A weak base will not react with a strong acid.
C)A strong base can be extremely corrosive.
D)A strong base will readily accept protons from even weak acids.
E)All of the above are untrue.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
40
For the following acid-base reaction, identify what compound is formed in the space marked. HNO3 + KOH ⇌ ???? + H2O
A)KNO3
B)H3ONO3
C)KOH2
D)KOH2NO3
E)none of the above
A)KNO3
B)H3ONO3
C)KOH2
D)KOH2NO3
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements describes an acidic solution?
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
42
Qualitatively, what happens to the hydroxide ion concentration if you decrease the hydronium ion concentration?
A)The concentration of OH- stays the same but the ratio OH-/H3O+ changes.
B)The concentration of OH- increases but the ratio stays the same.
C)The concentration of OH- increases.
D)none of the above
A)The concentration of OH- stays the same but the ratio OH-/H3O+ changes.
B)The concentration of OH- increases but the ratio stays the same.
C)The concentration of OH- increases.
D)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the above illustrations shows a neutral aqueous solution?
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
44
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-5M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-14
B)1 × 10-5
C)1 × 109
D)1 × 105
E)1 × 10-9
A)1 × 10-14
B)1 × 10-5
C)1 × 109
D)1 × 105
E)1 × 10-9

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following reactions illustrates an amphoteric compound?
A)2 HF ⇌ H2F+ + F-
B)NaOH + HBr ⇌ NaBr + H2O
C)2 H2 + O2 ⇌ 2 H2O
D)All are amphoteric.
E)none of the above
A)2 HF ⇌ H2F+ + F-
B)NaOH + HBr ⇌ NaBr + H2O
C)2 H2 + O2 ⇌ 2 H2O
D)All are amphoteric.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
46
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-1M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-3
B)1 × 10-2
C)1 × 10-4
D)1 × 1013
E)none of the above
A)1 × 10-3
B)1 × 10-2
C)1 × 10-4
D)1 × 1013
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
47
As the hydronium ion concentration increases, the pH ________.
A)goes down
B)gets larger
C)starts to affect the OH- concentration
D)starts to alter the chemical properties of the water molecules
E)stays constant
A)goes down
B)gets larger
C)starts to affect the OH- concentration
D)starts to alter the chemical properties of the water molecules
E)stays constant

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
48
What do the brackets in the following equation represent? [H3O+] × [OH-] = Kw
A)The brackets mean the molarity of the compound inside them.
B)The molecule inside does not react with the other molecules in brackets.
C)The molecule in brackets is undergoing hydrogen bonding with the other molecule in brackets.
D)The first molecule in brackets is an acid and it reacts with the second molecule in brackets, which is a base.
E)none of the above
A)The brackets mean the molarity of the compound inside them.
B)The molecule inside does not react with the other molecules in brackets.
C)The molecule in brackets is undergoing hydrogen bonding with the other molecule in brackets.
D)The first molecule in brackets is an acid and it reacts with the second molecule in brackets, which is a base.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
49
If the pH of a solution is 10, what is the hydronium ion concentration?
A)1 × 10-10 M
B)1 × 1010 M
C)10 M
D)-10 M
E)- 1 × 1010 M
A)1 × 10-10 M
B)1 × 1010 M
C)10 M
D)-10 M
E)- 1 × 1010 M

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the above illustrations shows an acidic aqueous solution?
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
51
Arrange the following images of an aqueous base solution in order of increasing base strength: 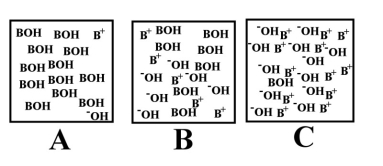
A)A, B, C
B)B, C, A
C)C, B, A
D)A, C, B
E)All are equally strong.

A)A, B, C
B)B, C, A
C)C, B, A
D)A, C, B
E)All are equally strong.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
52
What would the concentration of H3O+ be if the concentration of OH- was 1 × 10-11M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-3
B)1 × 103
C)1 × 1014
D)1 × 1012
E)1 × 10-6
A)1 × 10-3
B)1 × 103
C)1 × 1014
D)1 × 1012
E)1 × 10-6

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
53
Qualitatively, what happens to the hydronium ion concentration if you increase the hydroxide ion concentration?
A)The concentration of H3O+ stays the same but the ratio changes.
B)The concentration of H3O+ increases but the ratio stays the same.
C)The concentration of H3O+ increases to neutralize the excess hydroxide.
D)none of the above
A)The concentration of H3O+ stays the same but the ratio changes.
B)The concentration of H3O+ increases but the ratio stays the same.
C)The concentration of H3O+ increases to neutralize the excess hydroxide.
D)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
54
What would the concentration of OH- be if the concentration of H3O+ was 1 × 10-8M? [H3O+] × [OH-] = Kw = 1 × 10-14
A)1 × 10-14
B)1 × 10-6
C)1 × 108
D)1 × 106
E)1 × 10-8
A)1 × 10-14
B)1 × 10-6
C)1 × 108
D)1 × 106
E)1 × 10-8

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the above illustrations shows a basic aqueous solution?
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above
A)A
B)B
C)C
D)All of the above are neutral solutions.
E)none of the above

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
56
Sodium hydroxide, NaOH, is a strong base, which means that it readily accepts hydrogen ions. What products are formed when sodium hydroxide accepts a hydrogen ion from a water molecule?
A)water and sodium hydroxide
B)sodium hydroxide and hydronium ions
C)sodium ions and hydronium ions
D)sodium ions and water
A)water and sodium hydroxide
B)sodium hydroxide and hydronium ions
C)sodium ions and hydronium ions
D)sodium ions and water

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
57
A weak acid is added to a concentrated solution of hydrochloric acid. Does the solution become more or less acidic?
A)More acidic, since there are more hydronium ions being added to the solution.
B)Less acidic, since the solution becomes more dilute with a less concentrated solution of hydronium ions being added to the solution.
C)No change in acidity, since the concentration of the hydrochloric acid is too high to be changed by the weak solution.
D)Less acidic since the concentration of hydroxide ions will increase.
A)More acidic, since there are more hydronium ions being added to the solution.
B)Less acidic, since the solution becomes more dilute with a less concentrated solution of hydronium ions being added to the solution.
C)No change in acidity, since the concentration of the hydrochloric acid is too high to be changed by the weak solution.
D)Less acidic since the concentration of hydroxide ions will increase.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following solutions is the most acidic?
A)a solution with a pH = 14
B)a solution with a pH = 10
C)a solution with a pH = 7
D)a solution with a pH = 4
E)All of the solutions are basic.
A)a solution with a pH = 14
B)a solution with a pH = 10
C)a solution with a pH = 7
D)a solution with a pH = 4
E)All of the solutions are basic.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements describes a basic solution?
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following statements describes a neutral solution?
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1
A)[H3O+] > [OH-]
B)[H3O+] < [OH-]
C)[H3O+] × [OH-] ≠ 1 × 10-14
D)[H3O+]/[OH-] = 1 × 10-14
E)[H3O+]/[OH-] = 1

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
61
When a hydronium ion concentration equals 1 × 10-10 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
A)The pH of this solution is 4, which is acidic.
B)The pH of this solution is 8, which is basic.
C)The pH of this solution is 6, which is acidic.
D)The pH of this solution is 10, which is basic.
A)The pH of this solution is 4, which is acidic.
B)The pH of this solution is 8, which is basic.
C)The pH of this solution is 6, which is acidic.
D)The pH of this solution is 10, which is basic.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
62
If the pH of a solution was 7 and you were to increase the hydronium ion concentration 1000x, what would the pH be?
A)4
B)4 M
C)1 × 10-4 M
D)7,000 M
E)10
A)4
B)4 M
C)1 × 10-4 M
D)7,000 M
E)10

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
63
When the hydronium ion concentration equals 1 mole per liter, what is the pH of the solution? Is the solution acidic or basic?
A)pH = 0, this is an acidic solution.
B)pH = 1, this is an acidic solution.
C)pH = 10, this is a basic solution.
D)pH = 7, this is a neutral solution.
A)pH = 0, this is an acidic solution.
B)pH = 1, this is an acidic solution.
C)pH = 10, this is a basic solution.
D)pH = 7, this is a neutral solution.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
64
If you had a 1 M solution of a weak acid what would be its pH?
A)0
B)6
C)7
D)8
E)13
A)0
B)6
C)7
D)8
E)13

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
65
When a hydronium ion concentration equals 1 × 10-4 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
A)The pH is of this solution is 4 and it is acidic.
B)The pH is of this solution is 8 and it is basic.
C)The pH is of this solution is 6 and it is acidic.
D)The pH is of this solution is 10 and it is basic.
A)The pH is of this solution is 4 and it is acidic.
B)The pH is of this solution is 8 and it is basic.
C)The pH is of this solution is 6 and it is acidic.
D)The pH is of this solution is 10 and it is basic.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
66
What does the value of Kw say about the extent to which water molecule react with themselves?
A)Because the value of Kw is so small, the extent to which water ionizes is quite large.
B)Because the value of Kw is so small, the extent to which water ionizes is quite small.
C)Water molecules react with themselves at a constant rate, never increasing or decreasing.
D)When an acid is added to water, the value of Kw will decrease.
A)Because the value of Kw is so small, the extent to which water ionizes is quite large.
B)Because the value of Kw is so small, the extent to which water ionizes is quite small.
C)Water molecules react with themselves at a constant rate, never increasing or decreasing.
D)When an acid is added to water, the value of Kw will decrease.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
67
If the pH of a solution is 10, what is the hydroxide ion concentration?
A)1 × 10-4 M
B)4 M
C)14 M
D)-14 M
E)-1 × 1014 M
A)1 × 10-4 M
B)4 M
C)14 M
D)-14 M
E)-1 × 1014 M

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
68
If the pH of a solution was 7 and you were to increase the hydroxide ion concentration 100x, what would the pH be?
A)9
B)5 M
C)1 × 10-5 M
D)700 M
E)5
A)9
B)5 M
C)1 × 10-5 M
D)700 M
E)5

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
69
Why do we use the pH scale to indicate the acidity of a solution rather than simply stating the concentration of hydronium ions?
A)It includes the concentration of hydronium and hydroxide ions.
B)It is used because the general public understands it.
C)It is more accurate to use the pH scale.
D)It is more convenient, since the concentration of hydronium ions is so small.
A)It includes the concentration of hydronium and hydroxide ions.
B)It is used because the general public understands it.
C)It is more accurate to use the pH scale.
D)It is more convenient, since the concentration of hydronium ions is so small.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
70
Along with the pH scale, there is the pOH scale, which indicates the level of "basicity" in a solution. Accordingly, pOH = -log[OH-]. What is the sum of the pH and the pOH of a solution always equal to?
A)the negative of the product of the hydronium and hydroxide ions
B)the negative log of Kw, which is 14
C)The sum would equal 7, since that would be neutral for pH and pOH.
D)It is not constant because it depends on the acid/base solution in question.
A)the negative of the product of the hydronium and hydroxide ions
B)the negative log of Kw, which is 14
C)The sum would equal 7, since that would be neutral for pH and pOH.
D)It is not constant because it depends on the acid/base solution in question.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following solutions is the most acidic?
A)a solution with a pH = 14
B)a solution with a pH = 12
C)a solution with a pH = 7
D)a solution with a pH = 13
E)All of the solutions are basic.
A)a solution with a pH = 14
B)a solution with a pH = 12
C)a solution with a pH = 7
D)a solution with a pH = 13
E)All of the solutions are basic.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
72
If you had a 1 M solution of a weak base what would be its pH?
A)1
B)6
C)7
D)8
E)13
A)1
B)6
C)7
D)8
E)13

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
73
If you had a 1 M solution of a strong acid what would be its pH?
A)0
B)1
C)7
D)8
E)13
A)0
B)1
C)7
D)8
E)13

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
74
What happens to the pH of an acidic solution as water is added?
A)The pH is not influenced by the addition of water.
B)The pH will decrease as the solution becomes more dilute.
C)The pH will increase as the solution becomes more dilute.
D)The pH will decrease since more hydronium ions are produced from the water.
A)The pH is not influenced by the addition of water.
B)The pH will decrease as the solution becomes more dilute.
C)The pH will increase as the solution becomes more dilute.
D)The pH will decrease since more hydronium ions are produced from the water.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
75
What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × 10-10 moles per liter?
A)The concentration of hydroxide ions is 1 × 10-10 moles per liter.
B)The concentration of hydroxide ions is 1 × 10-4 moles per liter.
C)The concentration of hydroxide ions is 1 × 10-2 moles per liter.
D)The concentration of hydroxide ions is 1 × 10-6 moles per liter.
A)The concentration of hydroxide ions is 1 × 10-10 moles per liter.
B)The concentration of hydroxide ions is 1 × 10-4 moles per liter.
C)The concentration of hydroxide ions is 1 × 10-2 moles per liter.
D)The concentration of hydroxide ions is 1 × 10-6 moles per liter.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
76
What is the hydroxide ion concentration in an aqueous solution where the pH = 5?
A)The hydronium ion concentration equals 1 × 10-9.
B)The hydronium ion concentration equals 1 × 10-7.
C)The hydronium ion concentration equals 1 × 10-5.
D)The hydronium ion concentration equals 1 ×10-3.
A)The hydronium ion concentration equals 1 × 10-9.
B)The hydronium ion concentration equals 1 × 10-7.
C)The hydronium ion concentration equals 1 × 10-5.
D)The hydronium ion concentration equals 1 ×10-3.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
77
When the hydronium ion concentration equals 10 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
A)pH = 0, this is an acidic solution.
B)pH = 1, this is an acidic solution.
C)pH = -1, this is an acidic solution.
D)pH = 7, this is a neutral solution.
A)pH = 0, this is an acidic solution.
B)pH = 1, this is an acidic solution.
C)pH = -1, this is an acidic solution.
D)pH = 7, this is a neutral solution.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
78
The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + H2O + H2O → H3O+ + O H-
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of Kw be expected to increase, decrease, or stay the same with increasing temperature?
A)The value of Kw will stay the same, since it is a constant.
B)The value of Kw will decrease, since there are more hydronium ions than hydroxide ions.
C)The value of Kw will increase since the rise in temperature allows for a higher concentration of both ions.
D)The value of Kw will decrease since there are more hydroxide ions than hydronium ions.
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of Kw be expected to increase, decrease, or stay the same with increasing temperature?
A)The value of Kw will stay the same, since it is a constant.
B)The value of Kw will decrease, since there are more hydronium ions than hydroxide ions.
C)The value of Kw will increase since the rise in temperature allows for a higher concentration of both ions.
D)The value of Kw will decrease since there are more hydroxide ions than hydronium ions.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
79
What would be the concentration of hydronium ions in a solution that had a pH = -3? Why would such a solution be impossible to prepare?
A)Concentration of H3O+ = 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
B)Concentration of H3O+ = 30 M. It is impossible because pH can not be negative.
C)Concentration of H3O+ = 1030 M. It is impossible because any container used to hold the acid would immediately corrode.
D)Concentration of H3O+ = 103 M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.
A)Concentration of H3O+ = 3 M. It is impossible because it is hard to measure such a small amount of hydronium ions accurately.
B)Concentration of H3O+ = 30 M. It is impossible because pH can not be negative.
C)Concentration of H3O+ = 1030 M. It is impossible because any container used to hold the acid would immediately corrode.
D)Concentration of H3O+ = 103 M. It is impossible because only so much acid can dissolve in water before the solution becomes saturated.

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck
80
As the pH increases, the hydroxide ion concentration ________.
A)goes down
B)gets larger
C)starts to affect the H+ concentration
D)starts to decrease because it is reacting with the excess hydronium ions
E)stays constant
A)goes down
B)gets larger
C)starts to affect the H+ concentration
D)starts to decrease because it is reacting with the excess hydronium ions
E)stays constant

Unlock Deck
Unlock for access to all 182 flashcards in this deck.
Unlock Deck
k this deck


