Deck 17: How Chemicals React
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 17: How Chemicals React
1
Which equations are balanced? a)Mg (s)+ 2HCl (aq)→ MgCl2 (aq)+ H2 (g)
B)3Al (s)+ 3 Br2 (l)→ Al2Br3 (s)
C)2HgO (s)→ 2 Hg (l)+ O2 (g)
A)Only equation "c" is balanced.
B)Equations "a" and "c" are balanced.
C)Equations "b" and "c" are balanced.
D)All of them are balanced.
B)3Al (s)+ 3 Br2 (l)→ Al2Br3 (s)
C)2HgO (s)→ 2 Hg (l)+ O2 (g)
A)Only equation "c" is balanced.
B)Equations "a" and "c" are balanced.
C)Equations "b" and "c" are balanced.
D)All of them are balanced.
B
2
A friend argues that if mass were really conserved he would never need to refill his gas tank. What explanation do you offer your friend?
A)The atoms (mass)of gasoline are converted into energy by the engine according to E=m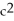 .
.
B)The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C)The atoms (mass)of gasoline are converted into exhaust fumes.
D)The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.
A)The atoms (mass)of gasoline are converted into energy by the engine according to E=m
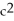 .
.B)The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C)The atoms (mass)of gasoline are converted into exhaust fumes.
D)The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.
C
3
Which of the following is a correctly balanced equation?
A)P4 + 6 H2 → 4 PH3
B)1 P4 + 6 H2 → 4 PH3
C)0 P4 + 6 H2 → 4 PH3
D)2 P4 + 12 H2 → 8 PH3
E)P4 + 3 H2 → PH3
A)P4 + 6 H2 → 4 PH3
B)1 P4 + 6 H2 → 4 PH3
C)0 P4 + 6 H2 → 4 PH3
D)2 P4 + 12 H2 → 8 PH3
E)P4 + 3 H2 → PH3
A
4
Steel wool wetted with vinegar is sealed within a balloon inflated with air. After several hours, what happens to the volume of the balloon?
A)The balloon inflates.
B)The balloon deflates.
C)The balloon dissolves.
D)Nothing because the vinegar is acting on the steel wool, not upon the balloon.
A)The balloon inflates.
B)The balloon deflates.
C)The balloon dissolves.
D)Nothing because the vinegar is acting on the steel wool, not upon the balloon.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
What coefficient is needed in front of the O2 molecule to balance the following equation? 2 C4H10 (g)+ _____ O2 (g)→ 8CO2 (g)+ 10 H2O (l)
A)8
B)13
C)5
D)1
A)8
B)13
C)5
D)1

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
What coefficients balance the following equation? ____ P4 (s)+ ____ H2 (g)→ ____ PH3 (g)
A)4, 2, 3
B)1, 6, 4
C)1, 4, 4
D)2, 10, 8
A)4, 2, 3
B)1, 6, 4
C)1, 4, 4
D)2, 10, 8

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
For the following balanced reaction, which of the following is a gas? 2 Na(l)+ Cl2(g)→ 2 NaCl(s)
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Steel wool wetted with vinegar is stuffed into a narrow mouth round glass bottle. A rubber balloon is then sealed over the mouth of the bottle. After several hours, the balloon inflates into the bottle in an inverted manner. What happened?
A)Vinegar fumes are diamagnetic and as they accumulate above the liquid the steel wool is attracted thus inflating the balloon into the mouth of the bottle in an inverted manner.
B)The caustic vinegar fumes get past the steel wool and deteriorate the balloon, which begins to sag into the bottle and inflate it in an inverted manner.
C)The vinegar reacts with the steel wool by absorbing oxygen within the bottle thus decreasing the pressure. The greater outside pressure causes the balloon to inflate in an inverted manner.
D)False! The balloon inflates above the mouth of the bottle because the reaction between the vinegar and steel wool produces a gas which is forced upward because of increased pressure inside the sealed bottle.
A)Vinegar fumes are diamagnetic and as they accumulate above the liquid the steel wool is attracted thus inflating the balloon into the mouth of the bottle in an inverted manner.
B)The caustic vinegar fumes get past the steel wool and deteriorate the balloon, which begins to sag into the bottle and inflate it in an inverted manner.
C)The vinegar reacts with the steel wool by absorbing oxygen within the bottle thus decreasing the pressure. The greater outside pressure causes the balloon to inflate in an inverted manner.
D)False! The balloon inflates above the mouth of the bottle because the reaction between the vinegar and steel wool produces a gas which is forced upward because of increased pressure inside the sealed bottle.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
What is a chemical reaction?
A)when one or more new compounds are formed by rearranging atoms
B)when a new element is formed by rearranging nucleons
C)when two solids mix together to form a heterogeneous mixture
D)when two liquids mix to form a homogeneous mixture
E)when a liquid undergoes a phase change and produces a solid
A)when one or more new compounds are formed by rearranging atoms
B)when a new element is formed by rearranging nucleons
C)when two solids mix together to form a heterogeneous mixture
D)when two liquids mix to form a homogeneous mixture
E)when a liquid undergoes a phase change and produces a solid

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
Given the following generic chemical reaction, which is the reactant? X → Y
A)Y is the reactant.
B)X is the reactant.
C)→ is the reactant.
D)Both X and Y are the products.
E)Both X and Y are the reactants.
A)Y is the reactant.
B)X is the reactant.
C)→ is the reactant.
D)Both X and Y are the products.
E)Both X and Y are the reactants.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
Balance the following chemical equation. ____ N2 + ____ H2 → ____ NH3
A)1, 3, 2
B)1, 2, 3
C)3, 2, 1
D)2, 6, 4
E)1/2, 3/2, 1
A)1, 3, 2
B)1, 2, 3
C)3, 2, 1
D)2, 6, 4
E)1/2, 3/2, 1

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Balance these equations. ____ H2 (g)+ ____ N2 (g)→ ____ NH3 (g)
A)2, 2, 3
B)2, 2, 5
C)3, 3, 2
D)3, 1, 2
A)2, 2, 3
B)2, 2, 5
C)3, 3, 2
D)3, 1, 2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
What is a chemical equation?
A)It is a shorthand notation for illustrating a chemical reaction.
B)It is the sum of the masses of the products and reactants.
C)It is the chemical combination of equal numbers of reactants and products.
D)It is a picture of the atoms undergoing a chemical equalization.
E)It is any type of reaction that takes place at the equator.
A)It is a shorthand notation for illustrating a chemical reaction.
B)It is the sum of the masses of the products and reactants.
C)It is the chemical combination of equal numbers of reactants and products.
D)It is a picture of the atoms undergoing a chemical equalization.
E)It is any type of reaction that takes place at the equator.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Given the following generic chemical reaction, which is the product? X → Y
A)Y is the product.
B)X is the product.
C)→ is the product.
D)Both X and Y are the products.
E)Both X and Y are the reactants.
A)Y is the product.
B)X is the product.
C)→ is the product.
D)Both X and Y are the products.
E)Both X and Y are the reactants.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
For the following balanced equation, which has the highest coefficient? 4 H2 + 2 C → 2 CH4
A)H2
B)C
C)CH4
D)H4
E)none of the above
A)H2
B)C
C)CH4
D)H4
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
For the following balanced reaction, which of the following is a solid? 2 Na(l)+ Cl2(g)→ 2 NaCl(s)
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
What is wrong with the following depiction of a chemical reaction? 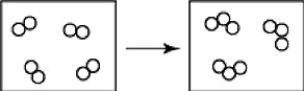
A)These boxes contain only molecules but no atoms.
B)One box contains more molecules than the other.
C)One box contains more atoms than the other.
D)All of the above.

A)These boxes contain only molecules but no atoms.
B)One box contains more molecules than the other.
C)One box contains more atoms than the other.
D)All of the above.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Which equation best describes the reaction represented in the illustration above?
A)2 AB2 + 2 DCB3 + B2 → 2 DBA4 + 2 CA2
B)2 AB2 + 2 CDA3 + B2 → 2 C2A4 + 2 DBA
C)2 AB2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2
D)2 BA2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2
A)2 AB2 + 2 DCB3 + B2 → 2 DBA4 + 2 CA2
B)2 AB2 + 2 CDA3 + B2 → 2 C2A4 + 2 DBA
C)2 AB2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2
D)2 BA2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
How many diatomic molecules are represented in the illustration above?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
Balance the following equation. ____ NO → ____ N2O + _____ NO2
A)3, 1, 1
B)3, 0, 0
C)4, 4, 8
D)1, 2, 4
E)6, 2, 1
A)3, 1, 1
B)3, 0, 0
C)4, 4, 8
D)1, 2, 4
E)6, 2, 1

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
The reactants shown schematically below represent iron oxide, Fe2O3 and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted? 
A)Fe2O3+ 3 CO → 2 Fe + 3 CO2
B)Fe2O3+ 3 CO → 3 FeO + 2 C
C)Fe2O3 + 3 CO → 3 FeO2 + 2 C
D)Fe2O3+ 3 CO → 2 Fe + 3 C2O

A)Fe2O3+ 3 CO → 2 Fe + 3 CO2
B)Fe2O3+ 3 CO → 3 FeO + 2 C
C)Fe2O3 + 3 CO → 3 FeO2 + 2 C
D)Fe2O3+ 3 CO → 2 Fe + 3 C2O

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
What is the formula mass of a molecule of CO2?
A)44 amu
B)56 amu
C)58.9 amu
D)118 amu
E)none of the above
A)44 amu
B)56 amu
C)58.9 amu
D)118 amu
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
How many oxygen molecules are needed to make 10 carbon dioxide molecules according to the following balanced chemical equation? 2 CO + O2 → 2 CO2
A)5
B)1
C)4
D)10
E)2
A)5
B)1
C)4
D)10
E)2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Is it possible to have a macroscopic sample of oxygen that has a mass of 14 atomic mass units?
A)Yes, but it would need to be made of oxygen atoms that each had less than the normal number of neutrons.
B)No, this is less than than the mass of a single oxygen atom.
C)Yes, but it would have the same density as nitrogen.
D)No, because oxygen is a gas at room temperature.
A)Yes, but it would need to be made of oxygen atoms that each had less than the normal number of neutrons.
B)No, this is less than than the mass of a single oxygen atom.
C)Yes, but it would have the same density as nitrogen.
D)No, because oxygen is a gas at room temperature.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Why is it important for a chemist to know the relative masses of atoms?
A)There are not that many different kinds of atoms and so it's important to know how they relate to one another.
B)It provides information about how many atoms two samples have relative to each other
C)It provides an indication of how the different atoms will interact
D)Because the mass of an atom is directly related to its chemical properties.
A)There are not that many different kinds of atoms and so it's important to know how they relate to one another.
B)It provides information about how many atoms two samples have relative to each other
C)It provides an indication of how the different atoms will interact
D)Because the mass of an atom is directly related to its chemical properties.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
You are given two samples of elements, and each sample has a mass of 10 grams. If the number of atoms in each of these samples is the same, what must be true of the two elements?
A)The density of the two elements are the same.
B)The elements are likely be located in the same position in the periodic table.
C)Their spectral patterns will likely be identical.
D)all of the above
A)The density of the two elements are the same.
B)The elements are likely be located in the same position in the periodic table.
C)Their spectral patterns will likely be identical.
D)all of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
How does formula mass differ from atomic mass?
A)They represent the same thing.
B)The formula mass of a substance is the sum of the atomic masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
C)The atomic mass of a substance is the sum of the formula masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
D)The formula mass is the mass of the chemical formula and the atomic mass is the mass of the molecule.
A)They represent the same thing.
B)The formula mass of a substance is the sum of the atomic masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
C)The atomic mass of a substance is the sum of the formula masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
D)The formula mass is the mass of the chemical formula and the atomic mass is the mass of the molecule.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
If it takes three golf balls to equal the mass of one tennis ball, what mass of tennis balls do you need to equal the number of golf balls in one kilogram of golf balls?
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
What is the formula mass of a molecule of C6H12O6?
A)180 amu
B)24 amu
C)29 amu
D)168 amu
E)none of the above
A)180 amu
B)24 amu
C)29 amu
D)168 amu
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
Which is greater: 1.01 amu of hydrogen or 1.01 grams of hydrogen?
A)1.01 amu of hydrogen is greater than 1.01 grams of hydrogen.
B)1.01 grams of hydrogen is greater than 1.01 amu of hydrogen
C)1.01 grams of hydrogen and 1.01 amu of hydrogen have the same mass.
D)Not enough information information is provided.
A)1.01 amu of hydrogen is greater than 1.01 grams of hydrogen.
B)1.01 grams of hydrogen is greater than 1.01 amu of hydrogen
C)1.01 grams of hydrogen and 1.01 amu of hydrogen have the same mass.
D)Not enough information information is provided.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
If it takes 200 golf balls to equal the mass of four bowling balls, what is the relative mass of bowling balls to golf balls?
A)1/50
B)1/20
C)20 times
D)100 times
E)6.022 × 1023
A)1/50
B)1/20
C)20 times
D)100 times
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
The relative mass of carbon is 3/8 that of an oxygen molecule. How many grams of carbon are needed to have the same number of particles as found in 32 grams of oxygen molecules?
A)12 g
B)32 g
C)3 g
D)8 g
E)3/8 g
A)12 g
B)32 g
C)3 g
D)8 g
E)3/8 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
If it takes three carbon atoms to equal the mass of one chlorine atom, what weight of chlorine do you need to equal the number of atoms in one kilogram of carbon?
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
If the relative mass of a hydrogen atom is 1/4 that of a helium atom, how many hydrogen atoms would you need to equal the mass of four helium atoms?
A)16
B)4
C)1/4
D)25
E)6.022 × 1023
A)16
B)4
C)1/4
D)25
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
If the relative mass of a pingpong ball is 1/20 that of a golf ball, how many golf balls would you need to equal the mass of 200 pingpong balls?
A)10
B)200
C)100
D)20
E)6.022 × 1023
A)10
B)200
C)100
D)20
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
What are the formula masses of water, H2O; propene, C3H6; and 2-propanol, C3H8O?
A)water: 18 amu; propene: 40 amu; 2-propanol: 58 amu
B)water: 18 amu; propene: 42 amu; 2-propanol: 62 amu
C)water: 18 amu; propene: 42 amu; 2-propanol: 60 amu
D)water: 18 amu; propene: 44 amu; 2-propanol: 64 amu
A)water: 18 amu; propene: 40 amu; 2-propanol: 58 amu
B)water: 18 amu; propene: 42 amu; 2-propanol: 62 amu
C)water: 18 amu; propene: 42 amu; 2-propanol: 60 amu
D)water: 18 amu; propene: 44 amu; 2-propanol: 64 amu

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
If the relative mass of a pingpong ball is 1/20 that of a golf ball, how many pingpong balls would you need to equal the mass of two golf balls?
A)40
B)20
C)24
D)100
E)6.022 × 1023
A)40
B)20
C)24
D)100
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
What is the formula mass of sulfur dioxide, SO2?
A)about 16 amu
B)about 32 amu
C)about 60 amu
D)about 64 amu
A)about 16 amu
B)about 32 amu
C)about 60 amu
D)about 64 amu

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
If it takes 20 beryllium atoms to equal the mass of two krypton atoms, what is the relative mass of beryllium compared to krypton?
A)1/10
B)1/20
C)40 times
D)100 times
E)10 times
A)1/10
B)1/20
C)40 times
D)100 times
E)10 times

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
If the relative mass of a hydrogen atom is 1/4 that of a helium atom, how many helium atoms would you need to equal the mass of 200 hydrogen atoms?
A)50
B)200
C)800
D)4
E)100
A)50
B)200
C)800
D)4
E)100

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
According to the following balanced chemical equation, if you want to generate two moles of H2O how many grams of O2 do you need? 2 H2 + O2 → 2 H2O
A)32
B)16
C)8
D)4
E)6.022 × 1023
A)32
B)16
C)8
D)4
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
Two amu equals how many grams?
A)2 grams
B)1.661 × 10-24 grams
C)3.322 × 10-24 grams
D)1.204 × 10-22 grams
A)2 grams
B)1.661 × 10-24 grams
C)3.322 × 10-24 grams
D)1.204 × 10-22 grams

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Which has the greatest number of molecules?
A)28 g of nitrogen, N2
B)32 g of oxygen, O2
C)32 g of methane, C H4
D)38 g of fluorine, F2
A)28 g of nitrogen, N2
B)32 g of oxygen, O2
C)32 g of methane, C H4
D)38 g of fluorine, F2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
What is the mass of a water molecule, H2O, in atomic mass units?
A)2 amu
B)3 amu
C)16 amu
D)18 amu
A)2 amu
B)3 amu
C)16 amu
D)18 amu

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following has the greatest mass?
A)1 mole of Pb
B)1 mole of H2
C)1 mole of Be
D)1 mole of Na
E)All have the same mass.
A)1 mole of Pb
B)1 mole of H2
C)1 mole of Be
D)1 mole of Na
E)All have the same mass.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
How many grams of water can be produced from the combination of 25.0 grams of hydrogen and 225 grams of oxygen?
A)250 grams
B)225 grams
C)200 grams
D)25 grams
A)250 grams
B)225 grams
C)200 grams
D)25 grams

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
What is the number of grams of CO2 produced if you combust 0.50 mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)22 g
B)10 g
C)44 g
D)32 g
E)1 g
A)22 g
B)10 g
C)44 g
D)32 g
E)1 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
What is the mass of an oxygen atom, O, in atomic mass units?
A)12 amu
B)16 amu
C)18 amu
D)32 amu
A)12 amu
B)16 amu
C)18 amu
D)32 amu

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following has the greatest number of particles?
A)1 mole of Na
B)22.990 g of Na
C)1 mole of Be
D)9.012 g of Be
E)All are the same.
A)1 mole of Na
B)22.990 g of Na
C)1 mole of Be
D)9.012 g of Be
E)All are the same.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
How many grams of water can be formed from the reaction between 10 grams of oxygen and 1 gram of hydrogen?
A)11 grams of water are formed since mass must be conserved.
B)10 grams of water are formed since you can't get a greater mass of water produced than oxygen reacting.
C)9 grams of water are formed because oxygen and hydrogen react in an 8:1 ratio.
D)No water is formed because there is insufficient hydrogen to react with the oxygen.
A)11 grams of water are formed since mass must be conserved.
B)10 grams of water are formed since you can't get a greater mass of water produced than oxygen reacting.
C)9 grams of water are formed because oxygen and hydrogen react in an 8:1 ratio.
D)No water is formed because there is insufficient hydrogen to react with the oxygen.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
What is the mass of an oxygen atom, O, in grams?
A)16 grams
B)1.661 × 10-24 grams
C)2.66 × 10-23 grams
D)none of the above
A)16 grams
B)1.661 × 10-24 grams
C)2.66 × 10-23 grams
D)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
What is the number of molecules of O2 consumed if you combust one mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)1 molecule
B)2 molecules
C)6.022 × 1023 molecules
D)1.204 × 024 molecules
E)1 g of molecules
A)1 molecule
B)2 molecules
C)6.022 × 1023 molecules
D)1.204 × 024 molecules
E)1 g of molecules

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Which has the greatest number of atoms?
A)28 g of nitrogen, N2
B)32 g of oxygen, O2
C)16 g of methane, C H2
D)38 g of fluorine, F2
A)28 g of nitrogen, N2
B)32 g of oxygen, O2
C)16 g of methane, C H2
D)38 g of fluorine, F2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
According to the following balanced chemical equation, if you want to generate two moles of H2O , how many molecules of O2 do you need? 2 H2 + O2 → 2 H2O
A)1
B)2
C)1/2
D)4
E)6.022 × 1023
A)1
B)2
C)1/2
D)4
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
According to the following balanced chemical equation, if you want to generate two moles of H2O, how many moles of O2 do you need? 2 H2 + O2 → 2 H2O
A)1
B)2
C)1/2
D)4
E)6.022 × 1023
A)1
B)2
C)1/2
D)4
E)6.022 × 1023

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
What is the mass of one mole of H2?
A)2 g
B)1 g
C)20 g
D)6.022 × 1023 g
E)none of the above
A)2 g
B)1 g
C)20 g
D)6.022 × 1023 g
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
What is the number of moles of H2O produced if you combust one mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
What is the number of moles of H2O produced if you combust 0.5 mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
How many grams of water can be produced by the combination of 8 grams of oxygen and 8 grams of hydrogen?
A)16 grams
B)10 grams
C)9 grams
D)8 grams
A)16 grams
B)10 grams
C)9 grams
D)8 grams

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
How is Avogadro's number related to the numbers on the periodic table?
A)The atomic mass listed is the mass of Avogadro's number's worth of atoms.
B)The masses are all divisible by Avogadro's number, which gives you the weight of one mole.
C)The periodic table tells you the mass of one atom. From that, and Avogadro's number you know the number of moles.
D)The periodic table only gives us atomic numbers, not atomic mass.
E)The mass listed is Avogadro's number.
A)The atomic mass listed is the mass of Avogadro's number's worth of atoms.
B)The masses are all divisible by Avogadro's number, which gives you the weight of one mole.
C)The periodic table tells you the mass of one atom. From that, and Avogadro's number you know the number of moles.
D)The periodic table only gives us atomic numbers, not atomic mass.
E)The mass listed is Avogadro's number.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
What can you deduce about the activation energy of a reaction that takes billions of years to go to completion? How about a reaction that takes only fractions of a second?
A)The activation energy of both these reactions must be very low.
B)The activation energy of both these reactions must be very high.
C)The slow reaction must have a high activation energy while the fast reaction must have a low activation energy.
D)The slow reaction must have a low activation energy while the fast reaction must have a high activation energy.
A)The activation energy of both these reactions must be very low.
B)The activation energy of both these reactions must be very high.
C)The slow reaction must have a high activation energy while the fast reaction must have a low activation energy.
D)The slow reaction must have a low activation energy while the fast reaction must have a high activation energy.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
For the above energy profiles, which reaction has the lowest activation energy?
A)a
B)b
C)c
D)d
E)All have the same activation energy.
A)a
B)b
C)c
D)d
E)All have the same activation energy.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Is the synthesis of ozone, O3, from oxygen, O2, an example of an exothermic or endothermic reaction?
A)exothermic because ultraviolet light is emitted during its formation
B)endothermic because ultraviolet light is emitted during its formation
C)exothermic because ultraviolet light is absorbed during its formation
D)endothermic because ultraviolet light is absorbed during its formation
A)exothermic because ultraviolet light is emitted during its formation
B)endothermic because ultraviolet light is emitted during its formation
C)exothermic because ultraviolet light is absorbed during its formation
D)endothermic because ultraviolet light is absorbed during its formation

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
What is the mass of a water molecule, H2O, in grams?
A)18 grams
B)1.661 × 10-24 grams
C)2.99 × 10-23 grams
D)none of the above
A)18 grams
B)1.661 × 10-24 grams
C)2.99 × 10-23 grams
D)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
For the above energy profiles, which reaction has the highest activation energy?
A)a
B)b
C)c
D)d
E)All have the same activation energy.
A)a
B)b
C)c
D)d
E)All have the same activation energy.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
How many grams of gallium are there in a 145 gram sample of gallium arenside, GaAs?
A)74.9 g
B)69.7 g
C)145 g
D)6.02 × 1023 g
A)74.9 g
B)69.7 g
C)145 g
D)6.02 × 1023 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Why might increasing the temperature alter the rate of a chemical reaction?
A)The molecules will have a higher kinetic energy and bump into one another harder.
B)The molecules are less reactive at higher temperatures.
C)The molecules will more likely combine with other atoms at high temperature to save space.
D)The density decreases as a function of temperature and this leads to an increase in volume which drops the rate of reaction.
E)none of the above
A)The molecules will have a higher kinetic energy and bump into one another harder.
B)The molecules are less reactive at higher temperatures.
C)The molecules will more likely combine with other atoms at high temperature to save space.
D)The density decreases as a function of temperature and this leads to an increase in volume which drops the rate of reaction.
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Small samples of oxygen gas needed in the laboratory can be generated by any number of simple chemical reactions, such as 2 KCl O3 (s)→ 2 KCl (s)+ 3 O2 (g)
What mass of oxygen (in grams)is produced when 122.6 g of KCl O3 (formula mass = 122.6 amu)takes part in this reaction?
A)32.00 grams
B)48.00 grams
C)96.00 grams
D)More information is needed.
What mass of oxygen (in grams)is produced when 122.6 g of KCl O3 (formula mass = 122.6 amu)takes part in this reaction?
A)32.00 grams
B)48.00 grams
C)96.00 grams
D)More information is needed.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
An Alka-Seltzer antacid tablet bubbles vigorously when placed in water but only slowly when placed in an alcoholic beverage of the same temperature containing a 50:50 mix of alcohol and water. Propose a probable explanation involving the relationship between the speed of a reaction and molecular collisions.
A)The alcohol absorbs the carbon dioxide bubbles before they escape the liquid phase.
B)Alcohol molecules are more massive than water molecules, hence they move slower and their collisions are not as forceful.
C)The tablet reacts with water but not the alcohol.
D)In a 50:50 mix there are fewer water molecules for the antacid molecules to collide with.
A)The alcohol absorbs the carbon dioxide bubbles before they escape the liquid phase.
B)Alcohol molecules are more massive than water molecules, hence they move slower and their collisions are not as forceful.
C)The tablet reacts with water but not the alcohol.
D)In a 50:50 mix there are fewer water molecules for the antacid molecules to collide with.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
What is a reaction rate?
A)It is the speed at which reactants are consumed or product is formed.
B)It is the balanced chemical formula that relates the number of product molecules to reactant molecules.
C)It is the ratio of the masses of products and reactants.
D)It is the ratio of the molecular masses of the elements in a given compound.
E)none of the above
A)It is the speed at which reactants are consumed or product is formed.
B)It is the balanced chemical formula that relates the number of product molecules to reactant molecules.
C)It is the ratio of the masses of products and reactants.
D)It is the ratio of the molecular masses of the elements in a given compound.
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
The yeast in bread dough feeds on sugar to produce carbon dioxide. Why does the dough rise faster in a warmer area?
A)There is a greater number of effective collisions among reacting molecules.
B)Atmospheric pressure decreases with increasing temperature.
C)The yeast tends to "wake up" with warmer temperatures, which is why baker's yeast is best stored in the refrigerator.
D)The rate of evaporation increases with increasing temperature.
A)There is a greater number of effective collisions among reacting molecules.
B)Atmospheric pressure decreases with increasing temperature.
C)The yeast tends to "wake up" with warmer temperatures, which is why baker's yeast is best stored in the refrigerator.
D)The rate of evaporation increases with increasing temperature.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
What is the activation energy?
A)the minimum amount of energy to break the bonds in reactants
B)the amount of energy required to activate a phase change
C)the energy difference between the reactants and the products
D)the amount of energy required to separate reactants from the products
E)the hill
A)the minimum amount of energy to break the bonds in reactants
B)the amount of energy required to activate a phase change
C)the energy difference between the reactants and the products
D)the amount of energy required to separate reactants from the products
E)the hill

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Why is heat often added to chemical reactions performed in the laboratory?
A)to allow a greater number of reactants to pass over the activation energy
B)to increase the rate at which reactant collide
C)to compensate for the natural tendency of energy to disperse
D)all of the above
A)to allow a greater number of reactants to pass over the activation energy
B)to increase the rate at which reactant collide
C)to compensate for the natural tendency of energy to disperse
D)all of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Why does a glowing splint of wood burn only slowly in air, but rapidly in a burst of flames when placed in pure oxygen?
A)There is a greater number of collisions between the wood and oxygen molecules.
B)Oxygen is a flammable gas.
C)Pure oxygen is able to absorb carbon dioxide at a faster rate.
D)A glowing wood splint is actually extinguished within pure oxygen because there's no room for the smoke to expand.
A)There is a greater number of collisions between the wood and oxygen molecules.
B)Oxygen is a flammable gas.
C)Pure oxygen is able to absorb carbon dioxide at a faster rate.
D)A glowing wood splint is actually extinguished within pure oxygen because there's no room for the smoke to expand.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
How many grams of water, H2O, and propene, C3H6, can be formed from the reaction of 6.0 g of 2-propanol, C3H8O? C3H8O → C3H6 + H2O
2-Propanol Propene Water
A)6.0 grams of propene, 0.0 grams of water
B)1.8 grams of propene, 4.2 grams of water
C)0.0 grams of propene, 6.0 grams of water
D)4.2 grams of propene, 1.8 grams of water
2-Propanol Propene Water
A)6.0 grams of propene, 0.0 grams of water
B)1.8 grams of propene, 4.2 grams of water
C)0.0 grams of propene, 6.0 grams of water
D)4.2 grams of propene, 1.8 grams of water

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
A refrigerator delays the spoilage of food by
A)killing microorganisms.
B)slowing down the rate of chemical reactions within microorganisms.
C)expanding the water found within microorganisms.
D)diminishing the supply of oxygen to microorganisms.
A)killing microorganisms.
B)slowing down the rate of chemical reactions within microorganisms.
C)expanding the water found within microorganisms.
D)diminishing the supply of oxygen to microorganisms.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
Why might increasing the concentration of a set of reactants increase the rate of reaction?
A)You have increased the chances that any two reactant molecules will collide and react.
B)You have increased the ratio of reactants to products.
C)The concentration of reactants is unrelated to the rate of reaction.
D)The rate of reaction depends only on the mass of the atoms and therefore increases as you increase the mass of the reactants.
E)none of the above
A)You have increased the chances that any two reactant molecules will collide and react.
B)You have increased the ratio of reactants to products.
C)The concentration of reactants is unrelated to the rate of reaction.
D)The rate of reaction depends only on the mass of the atoms and therefore increases as you increase the mass of the reactants.
E)none of the above

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
How many moles of water, H2O, are produced from the reaction of 16 grams methane, CH4, with an unlimited supply of oxygen, O2. How many grams of H2O is this? CH4 + 2 O2 → CO2 + 2 H2O
A)0.889 mole, which is 16 grams
B)2.0 moles of water, which is 32 grams
C)2.0 moles of water, which is 36 grams
D)1.0 mole of water, which is 18 grams
A)0.889 mole, which is 16 grams
B)2.0 moles of water, which is 32 grams
C)2.0 moles of water, which is 36 grams
D)1.0 mole of water, which is 18 grams

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
A 1.00 carat pure diamond has a mass of 0.20 grams. How many carbon atoms are there within this diamond?
A)6.0 × 1023 carbon atoms
B)2.0 × 1022 carbon atoms
C)1.0 × 1022 carbon atoms
D)6.0 × 1022 carbon atoms
A)6.0 × 1023 carbon atoms
B)2.0 × 1022 carbon atoms
C)1.0 × 1022 carbon atoms
D)6.0 × 1022 carbon atoms

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
How many molecules of aspirin (formula mass aspirin = 180.0 amu)are there in a 0.250-gram sample?
A)6.02 × 1023
B)8.36 × 1020
C)1.51× 1023
D)More information is needed.
A)6.02 × 1023
B)8.36 × 1020
C)1.51× 1023
D)More information is needed.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


