Deck 40: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 40: Nuclear Physics
1
A certain radioactive isotope decays to one sixteenth of its original amount in 4.0 hours.What is its half-life?
A)1.0 hours
B)2.5 hours
C)2.0 hours
D)1.4 hours
A)1.0 hours
B)2.5 hours
C)2.0 hours
D)1.4 hours
1.0 hours
2
A radioactive atom undergoes - decay by emitting an electron and an anti-electron neutrino.When this occurs,what happens to the number of neutrons in the atom?
A)increases.
B)decreases.
C)remains constant.
D)One can't determine what happens to the number of neutrons using only the information given.
A)increases.
B)decreases.
C)remains constant.
D)One can't determine what happens to the number of neutrons using only the information given.
decreases.
3
A certain radioactive isotope decays to one fourth of its original amount in 4.0 hours.How long would it take for 20% of it to decay?
A)0.40 hours
B)0.56 hours
C)0.64 hours
D)0.85 hours
A)0.40 hours
B)0.56 hours
C)0.64 hours
D)0.85 hours
0.64 hours
4
A radioactive atom undergoes - decay by emitting an electron and an anti-electron neutrino.When this occurs,what happens to the number of protons in the atom?
A)increases.
B)decreases.
C)remains constant.
D)One can't determine what happens to the number of protons using only the information given.
A)increases.
B)decreases.
C)remains constant.
D)One can't determine what happens to the number of protons using only the information given.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
A certain radioactive isotope decays to one fourth of its original amount in 4.0 hours.What is its half life?
A)1.4 hours
B)2.0 hours
C)2.5 hours
D)3.4 hours
A)1.4 hours
B)2.0 hours
C)2.5 hours
D)3.4 hours

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
A drug containing 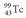 with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the half-life of
with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the half-life of 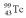 ?
?
A)6.05 hr
B)8.74 hr
C)12.6 hr
D)7.45 hr
E)6.91 hr
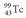 with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the half-life of
with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the half-life of 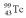 ?
?A)6.05 hr
B)8.74 hr
C)12.6 hr
D)7.45 hr
E)6.91 hr

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
An archeologist discovers the bones of a person believed to have been dead for 600 years.Using carbon dating,they wish to confirm the time of the person's demise.The rate of change of 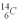 is 0.270 Bq/g of carbon for the bones of a person who had just died.If the archeologist is correct about the person's time of death,what rate of change of
is 0.270 Bq/g of carbon for the bones of a person who had just died.If the archeologist is correct about the person's time of death,what rate of change of 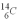 should they find when they carbon date the bones? The half life of
should they find when they carbon date the bones? The half life of 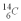 is 5.73 x 103 y.
is 5.73 x 103 y.
A)0.245 Bq/g
B)0.267 Bq/g
C)0.251 Bq/g
D)0.238 Bq/g
E)0.259 Bq/g
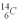 is 0.270 Bq/g of carbon for the bones of a person who had just died.If the archeologist is correct about the person's time of death,what rate of change of
is 0.270 Bq/g of carbon for the bones of a person who had just died.If the archeologist is correct about the person's time of death,what rate of change of 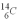 should they find when they carbon date the bones? The half life of
should they find when they carbon date the bones? The half life of 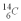 is 5.73 x 103 y.
is 5.73 x 103 y.A)0.245 Bq/g
B)0.267 Bq/g
C)0.251 Bq/g
D)0.238 Bq/g
E)0.259 Bq/g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
The rate of change of 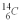 is 0.270 Bq per gram of carbon for the bones of a person who had just died.What is the decay constant? The half life of
is 0.270 Bq per gram of carbon for the bones of a person who had just died.What is the decay constant? The half life of 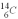 is 5.73 *103 y-1.
is 5.73 *103 y-1.
A)1.21 x 10-4 y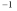
B)2.31 x 10-4 y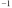
C)4.69 x 10-4 y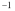
D)5.25 x 10-4 y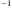
E)6.34 x 10-4 y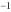
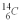 is 0.270 Bq per gram of carbon for the bones of a person who had just died.What is the decay constant? The half life of
is 0.270 Bq per gram of carbon for the bones of a person who had just died.What is the decay constant? The half life of 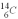 is 5.73 *103 y-1.
is 5.73 *103 y-1.A)1.21 x 10-4 y
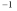
B)2.31 x 10-4 y
C)4.69 x 10-4 y
D)5.25 x 10-4 y
E)6.34 x 10-4 y

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
A radioactive atom emits an alpha particle.When this occurs,what happens to the mass number of the atom?
A)It increases.
B)It decreases.
C)It remains constant.
D)One can't determine what happens to the atomic number using only the information given.
A)It increases.
B)It decreases.
C)It remains constant.
D)One can't determine what happens to the atomic number using only the information given.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
A drug containing 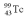 ( t 1/2 = 6.05 h)with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).What activity should the drug have at the preparation time (7:00 am)?
( t 1/2 = 6.05 h)with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).What activity should the drug have at the preparation time (7:00 am)?
A)0.25 Ci
B)1.34 Ci
C)2.87 Ci
D)3.16 Ci
E)4.47 Ci
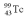 ( t 1/2 = 6.05 h)with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).What activity should the drug have at the preparation time (7:00 am)?
( t 1/2 = 6.05 h)with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).What activity should the drug have at the preparation time (7:00 am)?A)0.25 Ci
B)1.34 Ci
C)2.87 Ci
D)3.16 Ci
E)4.47 Ci

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
A certain radioactive isotope decays to one sixteenth of its original amount in 4.0 hours.What is its mean lifetime?
A)1.4 hours
B)2.0 hours
C)2.5 hours
D)3.4 hours
A)1.4 hours
B)2.0 hours
C)2.5 hours
D)3.4 hours

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Which elements have the most stable nuclei because they have the greatest binding energy per nucleon?
A)hydrogen and helium
B)krypton and barium
C)iron and nickel
D)uranium and plutonium
A)hydrogen and helium
B)krypton and barium
C)iron and nickel
D)uranium and plutonium

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
An archeologist discovers the bones of a person who appeared to have been dead a very long time.Using carbon dating,they determine that the rate of change of the 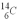 is 0.259 Bq per gram of carbon for the bones.The rate of change of
is 0.259 Bq per gram of carbon for the bones.The rate of change of 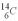 is 0.270 Bq per gram of carbon for the bones of a person who had just died.How old are the bones? The half life of
is 0.270 Bq per gram of carbon for the bones of a person who had just died.How old are the bones? The half life of 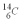 is 5.73 x 103 y.
is 5.73 x 103 y.
A)120 years
B)259 years
C)343 years
D)568 years
E)754 years
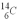 is 0.259 Bq per gram of carbon for the bones.The rate of change of
is 0.259 Bq per gram of carbon for the bones.The rate of change of 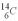 is 0.270 Bq per gram of carbon for the bones of a person who had just died.How old are the bones? The half life of
is 0.270 Bq per gram of carbon for the bones of a person who had just died.How old are the bones? The half life of 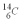 is 5.73 x 103 y.
is 5.73 x 103 y.A)120 years
B)259 years
C)343 years
D)568 years
E)754 years

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
A drug containing 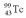 with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the decay constant of
with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the decay constant of 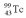 ?
?
A)0.165 hr-1
B)0.114 hr-1
C)0.0793 hr-1
D)0.204 hr-1
E)0.0961 hr-1
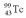 with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the decay constant of
with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the decay constant of 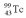 ?
?A)0.165 hr-1
B)0.114 hr-1
C)0.0793 hr-1
D)0.204 hr-1
E)0.0961 hr-1

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
A certain radioactive isotope has a half-life of 4.0 hours.How much time does it take for the amount of the isotope to decrease to one-eighth of its initial amount?
A)12 hours
B)8.0 hours
C)64 hours
D)16 hours
A)12 hours
B)8.0 hours
C)64 hours
D)16 hours

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
A nuclear fission power plant produces about 2.50 GW of electrical power.Assume that the plant has an overall efficiency of 40.0% and that each fission produces 200.0 MeV of energy.Calculate the mass of 235U consumed each day.
A)1.05 kg
B)2.63 kg
C)4.89 kg
D)6.58 kg
E)7.11 kg
A)1.05 kg
B)2.63 kg
C)4.89 kg
D)6.58 kg
E)7.11 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
The nucleus of a radioactive atom captures one of the atom's innermost electrons.When this occurs,what happens to the mass number of the atom?
A)It increases.
B)It decreases.
C)It remains constant.
D)One can't determine what happens to the atomic number using only the information given.
A)It increases.
B)It decreases.
C)It remains constant.
D)One can't determine what happens to the atomic number using only the information given.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
A drug containing 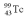 with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the mean lifetime of
with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the mean lifetime of 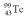 ?
?
A)6.05 hr
B)8.74 hr
C)12.6 hr
D)7.45 hr
E)6.91 hr
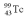 with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the mean lifetime of
with an activity of 2.00 Ci is to be injected into a patient at 11:00 am.A sample is prepared 4.00 hours before the injection (at 7:00 am).The activity of the drug have at the preparation time (7:00 am)was 3.16 Ci.What is the mean lifetime of 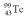 ?
?A)6.05 hr
B)8.74 hr
C)12.6 hr
D)7.45 hr
E)6.91 hr

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
A drug containing 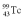 (
( 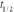 = 6.05 h)is to be injected into a patient at 10:00 am.You are to prepare the sample 3.00 hours before the injection (at 7:00 am)with an activity of 3.53 Ci at the preparation time (7:00 am).What will be the activity of the sample at the time of the injection?
= 6.05 h)is to be injected into a patient at 10:00 am.You are to prepare the sample 3.00 hours before the injection (at 7:00 am)with an activity of 3.53 Ci at the preparation time (7:00 am).What will be the activity of the sample at the time of the injection?
A)1.50 Ci
B)2.00 Ci
C)2.50 Ci
D)3.00 Ci
E)3.50 Ci
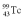 (
( 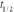 = 6.05 h)is to be injected into a patient at 10:00 am.You are to prepare the sample 3.00 hours before the injection (at 7:00 am)with an activity of 3.53 Ci at the preparation time (7:00 am).What will be the activity of the sample at the time of the injection?
= 6.05 h)is to be injected into a patient at 10:00 am.You are to prepare the sample 3.00 hours before the injection (at 7:00 am)with an activity of 3.53 Ci at the preparation time (7:00 am).What will be the activity of the sample at the time of the injection?A)1.50 Ci
B)2.00 Ci
C)2.50 Ci
D)3.00 Ci
E)3.50 Ci

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
Atoms consist of protons,neutrons,and electrons.Which determines an element of the periodic table?
A)the number of protons only
B)the number of neutrons only
C)the number of protons and neutrons
D)the number of protons,neutrons,and electrons
A)the number of protons only
B)the number of neutrons only
C)the number of protons and neutrons
D)the number of protons,neutrons,and electrons

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following fusion reaction 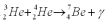 .The mass of
.The mass of 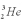 is 3.016029 u,the mass of
is 3.016029 u,the mass of 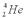 is 4.002602 u,and the mass of
is 4.002602 u,and the mass of 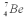 is 7.0169298 u.Assuming that the Be atom is at rest after the reaction,calculate the minimum possible energy of the photon g that is released in this reaction.
is 7.0169298 u.Assuming that the Be atom is at rest after the reaction,calculate the minimum possible energy of the photon g that is released in this reaction.
A)0.5 MeV
B)1 MeV
C)2 MeV
D)3 MeV
E)5 MeV
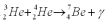 .The mass of
.The mass of 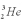 is 3.016029 u,the mass of
is 3.016029 u,the mass of 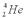 is 4.002602 u,and the mass of
is 4.002602 u,and the mass of 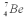 is 7.0169298 u.Assuming that the Be atom is at rest after the reaction,calculate the minimum possible energy of the photon g that is released in this reaction.
is 7.0169298 u.Assuming that the Be atom is at rest after the reaction,calculate the minimum possible energy of the photon g that is released in this reaction.A)0.5 MeV
B)1 MeV
C)2 MeV
D)3 MeV
E)5 MeV

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
A nuclear fission power plant consumes 7.11 kg of 235U each day to produce electrical power.Assume that the plant has an overall efficiency of 40.0% and that each fission produces 200.0 MeV of energy.Calculate the power output of the nuclear fission power plant.
A)2.90 GW
B)3.10 GW
C)2.70 GW
D)2.50 GW
E)2.30 GW
A)2.90 GW
B)3.10 GW
C)2.70 GW
D)2.50 GW
E)2.30 GW

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
The Sun has a mass of 1.99 * 1030 kg and radiates energy at a rate of 3.85 *1026 W.What is the change in mass of the Sun during one day?
A)1.6 * 1012 kg
B)2.2 * 1013 kg
C)8.7 * 1013 kg
D)3.7 * 1014 kg
E)1.1 * 1023 kg
A)1.6 * 1012 kg
B)2.2 * 1013 kg
C)8.7 * 1013 kg
D)3.7 * 1014 kg
E)1.1 * 1023 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the binding energy per nucleon for 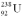 ,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.
,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.
A)9.03 * 10-11 J
B)8.52 * 10-11 J
C)2.65 * 10-12 J
D)1.21 *10-12 J
E)2.90 * 10-12 J
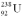 ,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.
,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.A)9.03 * 10-11 J
B)8.52 * 10-11 J
C)2.65 * 10-12 J
D)1.21 *10-12 J
E)2.90 * 10-12 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
Assuming a neutron star is as dense as an atomic nucleus and there are 7.1 * 1056 nucleons (almost entirely neutrons),estimate the diameter of this neutron star.
A)15 km
B)25 km
C)20 km
D)10 km
E)5 km
A)15 km
B)25 km
C)20 km
D)10 km
E)5 km

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
Assuming that a neutron star is as dense as an atomic nucleus,estimate the mass of a neutron star having a diameter of 10 km.
A)1.5 * 1029 kg
B)1.1 * 1029 kg
C)3.2 * 1029 kg
D)2.1 * 1029 kg
E)1.8 * 1029 kg
A)1.5 * 1029 kg
B)1.1 * 1029 kg
C)3.2 * 1029 kg
D)2.1 * 1029 kg
E)1.8 * 1029 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the binding energy of 7Li.(The mass of 7Li is 7.016004 u.)
A)12.34 MeV
B)23.45 MeV
C)39.25 MeV
D)54.32 MeV
E)64.35 MeV
A)12.34 MeV
B)23.45 MeV
C)39.25 MeV
D)54.32 MeV
E)64.35 MeV

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
What is the decay constant for 14C? Carbon 14 has a half-life of 5730 years?
A)1.2 * 10-4 s-1
B)5.3 * 10-5 s-1
C)1.7 * 10-12 s-1
D)3.8 * 10-12 s-1
E)5.5 * 10-12 s-1
A)1.2 * 10-4 s-1
B)5.3 * 10-5 s-1
C)1.7 * 10-12 s-1
D)3.8 * 10-12 s-1
E)5.5 * 10-12 s-1

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
In 1991 a frozen body of an "Iceman" was found in the Italian Alps.The fraction of 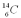 compared to
compared to 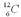 found in the body's bones,hair,and leather clothing was 6.94 * 10-13.The half-life of
found in the body's bones,hair,and leather clothing was 6.94 * 10-13.The half-life of 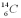 is 5,730 years and the ratio of
is 5,730 years and the ratio of 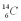 to
to 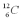 in living organisms is 1 to 7.41 *1011.When did the Iceman die?
in living organisms is 1 to 7.41 *1011.When did the Iceman die?
A)2500 years ago
B)3800 years ago
C)5500 years ago
D)7600 years ago
E)11,000 years ago
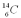 compared to
compared to 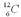 found in the body's bones,hair,and leather clothing was 6.94 * 10-13.The half-life of
found in the body's bones,hair,and leather clothing was 6.94 * 10-13.The half-life of 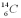 is 5,730 years and the ratio of
is 5,730 years and the ratio of 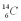 to
to 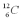 in living organisms is 1 to 7.41 *1011.When did the Iceman die?
in living organisms is 1 to 7.41 *1011.When did the Iceman die?A)2500 years ago
B)3800 years ago
C)5500 years ago
D)7600 years ago
E)11,000 years ago

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
Consider a hypothetical fission process where a 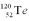 nucleus splits into two identical
nucleus splits into two identical 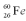 nuclei without producing any other particles or radiation.The mass of
nuclei without producing any other particles or radiation.The mass of 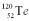 is 119.904040 u,and the mass of
is 119.904040 u,and the mass of 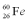 is 59.934078 u.At the moment when the two Fe nuclei form,but before they start moving away due to Coulomb repulsion,how far apart are the two Fe nuclei?
is 59.934078 u.At the moment when the two Fe nuclei form,but before they start moving away due to Coulomb repulsion,how far apart are the two Fe nuclei?
A)3 *10-14 m
B)7 * 10-14 m
C)4 * 10-13 m
D)6 * 10-10 m
E)2 * 10-7 m
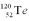 nucleus splits into two identical
nucleus splits into two identical 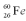 nuclei without producing any other particles or radiation.The mass of
nuclei without producing any other particles or radiation.The mass of 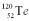 is 119.904040 u,and the mass of
is 119.904040 u,and the mass of 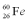 is 59.934078 u.At the moment when the two Fe nuclei form,but before they start moving away due to Coulomb repulsion,how far apart are the two Fe nuclei?
is 59.934078 u.At the moment when the two Fe nuclei form,but before they start moving away due to Coulomb repulsion,how far apart are the two Fe nuclei?A)3 *10-14 m
B)7 * 10-14 m
C)4 * 10-13 m
D)6 * 10-10 m
E)2 * 10-7 m

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
The fusion reaction: 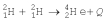 releases 23.85 MeV of energy.World power consumption is about 1013 W and 0.0300% of ocean water contains deuterium,
releases 23.85 MeV of energy.World power consumption is about 1013 W and 0.0300% of ocean water contains deuterium, 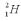 .If the deuterium in the ocean water were fused by controlled fusion with 100% efficiency,how much water would be needed every second to cover world's power consumption?
.If the deuterium in the ocean water were fused by controlled fusion with 100% efficiency,how much water would be needed every second to cover world's power consumption?
A)0.159 kg
B)2.32 kg
C)58.3 kg
D)522 kg
E)9470 kg
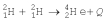 releases 23.85 MeV of energy.World power consumption is about 1013 W and 0.0300% of ocean water contains deuterium,
releases 23.85 MeV of energy.World power consumption is about 1013 W and 0.0300% of ocean water contains deuterium, 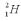 .If the deuterium in the ocean water were fused by controlled fusion with 100% efficiency,how much water would be needed every second to cover world's power consumption?
.If the deuterium in the ocean water were fused by controlled fusion with 100% efficiency,how much water would be needed every second to cover world's power consumption?A)0.159 kg
B)2.32 kg
C)58.3 kg
D)522 kg
E)9470 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
If protons decay with a half-life of 1030 years,how long will it take for 1% of the protons in the universe to decay?
A)6.3 * 1027 yr
B)1.0 * 1028 yr
C)1.5 * 1028 yr
D)9.9 * 1031 yr
E)1.1 * 1032 yr
A)6.3 * 1027 yr
B)1.0 * 1028 yr
C)1.5 * 1028 yr
D)9.9 * 1031 yr
E)1.1 * 1032 yr

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
A Geiger counter initially records 9876 counts per second.After 45 minutes it records 1234 counts/s.Ignore any uncertainty in the counts and find the half-life of the material.
A)900 s
B)950 s
C)1300 s
D)1350 s
E)2072 s
A)900 s
B)950 s
C)1300 s
D)1350 s
E)2072 s

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
if the mass of a star changes at a rate of 8.7 * 1013 kg/day,at what rate does the star radiate energy?
A)2.32 * 1026 W
B)3.85 * 1026 W
C)1.12 * 1026 W
D)8.12 * 1025 W
E)9.06 * 1025 W
A)2.32 * 1026 W
B)3.85 * 1026 W
C)1.12 * 1026 W
D)8.12 * 1025 W
E)9.06 * 1025 W

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
Element X decays to element Y via beta decay with a half-life of 0.77 s.Initially there is a sample of pure X and after 2.31 s there are 7 g of Y.What was the mass of the original sample?
A)1 g
B)7 g
C)8 g
D)14 g
E)56 g
A)1 g
B)7 g
C)8 g
D)14 g
E)56 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
Which isotope of carbon is the most stable?
A)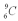
B)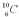
C)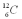
D)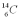
E)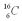
A)
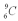
B)
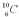
C)
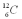
D)
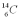
E)
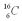

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
Assuming that a neutron star is as dense as an atomic nucleus,estimate the number of nucleons in a 10 km diameter star.
A)3.2* 1053
B)4.5 * 1054
C)8.9*1055
D)7.1 * 1056
E)2.1 *1057
A)3.2* 1053
B)4.5 * 1054
C)8.9*1055
D)7.1 * 1056
E)2.1 *1057

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the binding energy for 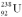 ,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.
,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.
A)2.65 *10-10 J
B)2.45 * 10-10 J
C)2.90 * 10-10 J
D)1.03 * 10-12 J
E)2.65 * 10-13 J
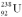 ,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.
,which consists of 92 protons,92 electrons,and 146 neutrons with a total mass of 238.0507826 u.A)2.65 *10-10 J
B)2.45 * 10-10 J
C)2.90 * 10-10 J
D)1.03 * 10-12 J
E)2.65 * 10-13 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
A nuclear fission power plant produces about 2.50 GW of electrical power.Assume that the plant has an overall efficiency of 40.0% and that each fission produces 200.0 MeV of energy.Calculate the number of 235U fissions required each day.
A)6.75 * 1024
B)3.12 * 1025
C)2.41 * 1025
D)2.89 * 1025
E)1.69 * 1025
A)6.75 * 1024
B)3.12 * 1025
C)2.41 * 1025
D)2.89 * 1025
E)1.69 * 1025

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
A nuclear fission power plant produces about 2.00 GW of electrical power.Assume that that each fission produces 200.0 MeV of energy and that the mass of 235U consumed each day is 5.50 kg.The overall efficiency of the plant is
A)32.4%.
B)38.3%.
C)41.5%.
D)46.2%.
E)51.7%.
A)32.4%.
B)38.3%.
C)41.5%.
D)46.2%.
E)51.7%.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
A radioactive isotope has a lifetime of 6.30 min.If you begin with a sample of 2.50 moles of this isotope,how many atoms have you left after one hour?
A)1.10 * 1020
B)4.5 * 1014
C)2.05 * 1021
D)None are correct.
A)1.10 * 1020
B)4.5 * 1014
C)2.05 * 1021
D)None are correct.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
What is the total energy released in the following decay? 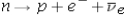
A)5 MeV
B)2 MeV
C)0.8 MeV
D)0.2 MeV
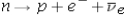
A)5 MeV
B)2 MeV
C)0.8 MeV
D)0.2 MeV

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
Assuming that 200 MeV of energy is released per fission,calculate the total energy released in the fission of 1 g of 235U.
A)2.28 * 104 kW h.
B)4.98 * 104 kW h.
C)2.28 * 105 kW h.
D)1.49 * 106 kW h.
A)2.28 * 104 kW h.
B)4.98 * 104 kW h.
C)2.28 * 105 kW h.
D)1.49 * 106 kW h.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
If 1030 atoms of a radioactive sample remain after 10 half-lives,how many atoms remain after 20 half-lives?
A)1029
B)1027
C)1026
D)1028
A)1029
B)1027
C)1026
D)1028

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
How large is the electrostatic repulsion between two protons a distance d = 6.7 fm apart in a nucleus?
A)7.5 N
B)75.5 N
C)48.4 N
D)8.95 N
E)5.13 N
A)7.5 N
B)75.5 N
C)48.4 N
D)8.95 N
E)5.13 N

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
A certain radioactive isotope has a lifetime of 343.6 s.Suppose you have a beam made of radioactive ions of this isotope travelling at 5.21 * 107 m/s in the laboratory,circulating in a cyclotron.If you begin with a sample of 2.50 micro-moles of this isotope,how many atoms have you left after one hour?
A)4.97 * 1013
B)4.23 * 1013
C)3.61 * 1013
D)None are correct.
A)4.97 * 1013
B)4.23 * 1013
C)3.61 * 1013
D)None are correct.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
131I,which has a half- life time of 8 days,is used in nuclear medicine to monitor the hormone production in the thyroid in a patient.A sample of 131I containing 5 * 1010 atoms is produced in a nuclear reactor.Due to the short half- life time,the sample emits less radiation with time.However,it is usable as long as there are at least 1.25 * 1010 131I atoms present.What is the maximum time before the sample is no longer usable?
A)16 days
B)12 days
C)8 days
D)6 days
A)16 days
B)12 days
C)8 days
D)6 days

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
What is the binding energy per nucleon of 56Fe? The atomic mass of 56Fe is 55.9349 u,the mass of a proton = 1.0078 u,the mass of a neutron = 1.0087 u,the atomic number Z = 26 and the atomic mass A = 56.
A)9.01 MeV
B)8.79 MeV
C)7.95 MeV
D)7.22 MeV
A)9.01 MeV
B)8.79 MeV
C)7.95 MeV
D)7.22 MeV

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
What is the binding energy of 56Fe? The atomic mass of 56Fe is 55.9349 u,the mass of a proton = 1.0078 u,the mass of a neutron = 1.0087 u,the atomic number Z = 26 and the atomic mass A = 56.
A)231 MeV
B)350 MeV
C)492 MeV
D)567 MeV
A)231 MeV
B)350 MeV
C)492 MeV
D)567 MeV

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
If you begin with a sample of 2.50 moles of a radioactive isotope and there are 2.05 * 1021 atoms left after one hour,what is the half-life of this isotope?
A)9.1 minutes
B)6.3 minutes
C)13 minutes
D)7.5 minutes
A)9.1 minutes
B)6.3 minutes
C)13 minutes
D)7.5 minutes

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
What is the surface area,approximately,of a Lead-208 nucleus (Z = 82)?
A)550 fm2
B)140 fm2
C)440 fm2
D)74 fm2
E)120 fm2
A)550 fm2
B)140 fm2
C)440 fm2
D)74 fm2
E)120 fm2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
The half-life of a sample of 1011 atoms that decay by alpha emission is 10 minutes.How many alpha particles are emitted between the time interval 100 min and 200 min?
A)9.8 * 107
B)9.8 * 104
C)2.0 * 106
D)1.0 * 107
A)9.8 * 107
B)9.8 * 104
C)2.0 * 106
D)1.0 * 107

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
If you begin with a sample of 2.50 moles of a radioactive isotope and there are 2.05 * 1021 atoms left after one hour,what is the mean lifetime of this isotope?
A)8.4 minutes
B)7.5 minutes
C)6.3 minutes
D)9.1 minutes
A)8.4 minutes
B)7.5 minutes
C)6.3 minutes
D)9.1 minutes

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
3.25 mole of a radioactive isotope with a half-life of 18.2 hours sits on a countertop for 2.7 days exactly.How much of the isotope is left?
A)0.14 mole
B)0.28 mole
C)1.34 mole
D)2.47 mole
E)3.25 mole
A)0.14 mole
B)0.28 mole
C)1.34 mole
D)2.47 mole
E)3.25 mole

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
In neutron stars,which are roughly 90% neutrons and supported almost entirely by nuclear forces,which of the following binding energy terms becomes relatively dominant compared to ordinary nuclei?
A)the coulomb term
B)the asymmetry term
C)the pairing term
D)All are correct.
E)None are correct.
A)the coulomb term
B)the asymmetry term
C)the pairing term
D)All are correct.
E)None are correct.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is true about strong force?
A)is only attractive
B)does not act on electrons
C)only acts over a few fm
D)All are correct.
A)is only attractive
B)does not act on electrons
C)only acts over a few fm
D)All are correct.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
If the Fermi energy of a neutron inside a nucleus is 38 MeV,estimate the maximum speed with which it moves around inside the nucleus.
A)90% of speed of light
B)10% of speed of light
C)0.1% of speed of light
D)30% of speed of light
A)90% of speed of light
B)10% of speed of light
C)0.1% of speed of light
D)30% of speed of light

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
If the Fermi energy of a neutron inside a nucleus is 38 MeV,what is the Fermi momentum?
A)38 MeV/c
B)270 MeV/c
C)370 MeV/c
D)154 MeV/c
A)38 MeV/c
B)270 MeV/c
C)370 MeV/c
D)154 MeV/c

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
A radioactive isotope has a lifetime of 6.30 min.If you begin with a sample of this isotope and there are 2.05 * 1021 atoms left after one hour,how many moles were in your initial sample?
A)2.50
B)46.6
C)17.2
D)98.5
A)2.50
B)46.6
C)17.2
D)98.5

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following would you expect to decay via b+ decay?
A)12C
B)132Sn
C)56Fe
D)74Kr
E)17N
A)12C
B)132Sn
C)56Fe
D)74Kr
E)17N

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
A radioactive material has a half life of 20 seconds.If the initial amount of material is G grams,how much will be left at the end of one minute?
A)G/3
B)G/4
C)G/6
D)G/8
E)G/16
A)G/3
B)G/4
C)G/6
D)G/8
E)G/16

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
When 21583Bi decays to 21584Po,what particle is released?
A)proton
B)neutron
C)positron
D)electron
E)alpha
A)proton
B)neutron
C)positron
D)electron
E)alpha

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
What particle has 9 protons,10 neutrons,and 11 electrons?
A)10F-2
B)19F-2
C)9Ne-2
D)19Ne-2
E)9K-2
A)10F-2
B)19F-2
C)9Ne-2
D)19Ne-2
E)9K-2

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
A Geiger counter placed next to a radioactive sample produces 200 counts per minute.One hour later,the Geiger counter reads 180 counts per minute.What is the half-life of the radioactive sample?
A)1.1 hours
B)6.6 hours
C)9.0 hours
D)9.5 hours
E)10 hours
A)1.1 hours
B)6.6 hours
C)9.0 hours
D)9.5 hours
E)10 hours

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
Sirius,the brightest star in the night sky coverts 3.216 * 1018 kg of its mass to energy every year.What is the luminosity (power radiated)of Sirius?
A)3.91 * 1026 W
B)1.09 * 1027 W
C)1.33 * 1028 W
D)5.23 * 1027 W
E)9.18 * 1027 W
A)3.91 * 1026 W
B)1.09 * 1027 W
C)1.33 * 1028 W
D)5.23 * 1027 W
E)9.18 * 1027 W

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
In a - decay process, 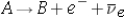 .If A has mass 14.0032235 u and B has mass 14.0031592 u,what is the maximum energy of the emitted electron?
.If A has mass 14.0032235 u and B has mass 14.0031592 u,what is the maximum energy of the emitted electron?
A)0.26 keV
B)12.4 keV
C)59.9 keV
D)231keV
E)4186 keV
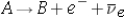 .If A has mass 14.0032235 u and B has mass 14.0031592 u,what is the maximum energy of the emitted electron?
.If A has mass 14.0032235 u and B has mass 14.0031592 u,what is the maximum energy of the emitted electron?A)0.26 keV
B)12.4 keV
C)59.9 keV
D)231keV
E)4186 keV

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
When carbon-14 undergoes a beta decay,an isotope of what element is produced?
A)beryllium
B)boron
C)carbon
D)nitrogen
E)oxygen
A)beryllium
B)boron
C)carbon
D)nitrogen
E)oxygen

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
U-238 has a half-life of 4.5 billion years and a present day abundance of 99.3%.U-235 has a half-life of 0.7 billion years and a present day abundance of 0.7%.Assuming a steady rate of decay,how long ago did these two isotopes have the same abundance?
A)4.1 billion years ago
B)5.2 billion years ago
C)5.9 billion years ago
D)8.4 billion years ago
E)9.9 billion years ago
A)4.1 billion years ago
B)5.2 billion years ago
C)5.9 billion years ago
D)8.4 billion years ago
E)9.9 billion years ago

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
The volume of a nucleus is proportional to
A)the atomic number Z.
B)the atomic mass number
C)the neutron number N.
D)the number of electrons.
A)the atomic number Z.
B)the atomic mass number
C)the neutron number N.
D)the number of electrons.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
A muon has a mean lifetime of only 2.200 µs in its own rest frame.If it moves with a speed of v = 0.9994 c in a certain reference frame,what is its mean lifetime in that frame?
A)0.042 µs
B)2.2 µs
C)9.99 µs
D)63.5 µs
E)4200 µs
A)0.042 µs
B)2.2 µs
C)9.99 µs
D)63.5 µs
E)4200 µs

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
An iron isotope of iron,56Fe,has a binding energy per nucleon of 8.791 MeV.What is its mass? (The mass of an hydrogen atom is 1.007825 u,the mass of a neutron is 1.008665 u,and the atomic mass unit is 1 u = 931.5 MeV.The number of protons in the iron nucleus is 26.)
A)26.20345 u
B)30.23475 u
C)55.93494 u
D)56.46340 u
E)56.99186 u
A)26.20345 u
B)30.23475 u
C)55.93494 u
D)56.46340 u
E)56.99186 u

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
A tree is chopped down and burned to produce ash.The carbon isotopes in the ash are found to have a ratio for 14C to 12C of 1.3 * 10-12.Experimental tests on the 14C atoms reveal that 14C is a beta emitter with a half-life of 5730 years.At an archeological excavation,a skeleton is found next to a campfire with some wood ash.If 100 g of carbon from the ash in the campfire emits betas at a rate of 40 per hour,how old is the campfire?
A)44,000 years
B)64,000 years
C)98,000 years
D)230,000 years
E)4,500,000 years
A)44,000 years
B)64,000 years
C)98,000 years
D)230,000 years
E)4,500,000 years

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Betelgeuse has a luminosity (power radiated)135,000 times that of the Sun,which emits energy at the rate of 3.906 * 1026 W.How much of its mass is being converted to energy in each year?
A)1.85 * 1022 kg
B)7.70 * 1020 kg
C)1.37 * 1017 kg
D)5.85 * 1014 kg
E)5.06 * 1019 kg
A)1.85 * 1022 kg
B)7.70 * 1020 kg
C)1.37 * 1017 kg
D)5.85 * 1014 kg
E)5.06 * 1019 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
When 21083Bi decays its half life is 5.0 seconds.What is the activity in Bq (disintegrations per second)of one mole?
A)6.02 * 1023/5
B)0.69 * 6.02 * 1023/5
C)5 * 6.02 * 1023
D)6.02 * 1023/5 * 0.69
E)5 * 6.02 * 1023/0.69
A)6.02 * 1023/5
B)0.69 * 6.02 * 1023/5
C)5 * 6.02 * 1023
D)6.02 * 1023/5 * 0.69
E)5 * 6.02 * 1023/0.69

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
The half-life of Co-57 is 271.8 days;calculate the decay constant.
A)11.2 * 10-8 s-1
B)16.0 * 10-4 s-1
C)2.95 * 108 s-1
D)3.0 * 10-8 s-1
E)6.3 * 106 s-1
A)11.2 * 10-8 s-1
B)16.0 * 10-4 s-1
C)2.95 * 108 s-1
D)3.0 * 10-8 s-1
E)6.3 * 106 s-1

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
Sirius,the brightest star in the night sky coverts 3.216 * 1018 kg of its mass to energy every year.How does the luminosity (power radiated)of Sirius compare to that of the sun? Lsun = 3.906 * 1026 W
A)17.6Lsun
B)23.5Lsun
C)3.56Lsun
D)36.7Lsun
E)42.0Lsun
A)17.6Lsun
B)23.5Lsun
C)3.56Lsun
D)36.7Lsun
E)42.0Lsun

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
If Carbon-14,a beta emitter with a half-life of 5730 years,makes up one out of 1012 Carbon atoms in nature,how many decays per second are there in a 15 kg bag of charcoal (taken to be 100% carbon)?
A)1720
B)2010
C)2480
D)2890
E)None are correct.
A)1720
B)2010
C)2480
D)2890
E)None are correct.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


