Deck 5: Proteins: Primary Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 5: Proteins: Primary Structure
1
Matching
______ chromatography is a method of fractionating a protein mixture according to differences in polarity.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
______ chromatography is a method of fractionating a protein mixture according to differences in polarity.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
hydrophobic interaction
2
Matching
Either dansyl chloride or Edman's reagent can be used to identify the ______ of a protein.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
Either dansyl chloride or Edman's reagent can be used to identify the ______ of a protein.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
N-terminal amino acid
3
The vast majority of polypeptides contain between ______ amino acid residues.
A)10 and 50
B)50 and 100
C)100 and 1000
D)1000 and 2000
E)2000 and 34,000
A)10 and 50
B)50 and 100
C)100 and 1000
D)1000 and 2000
E)2000 and 34,000
100 and 1000
4
Since there are 20 standard amino acids, the number of possible linear polypeptides of length N can be expressed as:
A)n × 20
B)20n
C)20 × 10n
D)n20
E)n × 1020
A)n × 20
B)20n
C)20 × 10n
D)n20
E)n × 1020

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
Which statement about insulin is correct?
A)Insulin is composed of two polypeptides, the A chain and the B chain.
B)Insulin contains an intrachain disulfide bond.
C)Insulin contains interchain disulfide bonds.
D)The A chain and the B chain of insulin are encoded by a single gene.
E)All of the above are correct.
A)Insulin is composed of two polypeptides, the A chain and the B chain.
B)Insulin contains an intrachain disulfide bond.
C)Insulin contains interchain disulfide bonds.
D)The A chain and the B chain of insulin are encoded by a single gene.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
Matching
The endoprotease ______ cleaves polypeptides on the C-terminal side of certain bulky hydrophobic amino acid residues.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
The endoprotease ______ cleaves polypeptides on the C-terminal side of certain bulky hydrophobic amino acid residues.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Natural proteins most commonly contain linear polypeptides between 100 and 1000 residues in length.One of the reasons polypeptides outside this range may be disfavored is that
A)larger polypeptides would likely be insoluble.
B)smaller polypeptides do not form stable folded structures.
C)smaller polypeptides typically assemble into prion-like aggregates.
D)amide linkages are not strong enough to keep larger polypeptides intact.
E)ribosomes are unable to synthesize larger polypeptides.
A)larger polypeptides would likely be insoluble.
B)smaller polypeptides do not form stable folded structures.
C)smaller polypeptides typically assemble into prion-like aggregates.
D)amide linkages are not strong enough to keep larger polypeptides intact.
E)ribosomes are unable to synthesize larger polypeptides.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Matching
If antibodies to the protein being assayed are available, a(n)______ can be carried out.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
If antibodies to the protein being assayed are available, a(n)______ can be carried out.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Matching
In general, proteins are least soluble in water when the pH is close to the ______.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
In general, proteins are least soluble in water when the pH is close to the ______.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
Matching
To help prevent denaturation of proteins in solution, steps are taken to avoid _________ and adsorption to surfaces.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
To help prevent denaturation of proteins in solution, steps are taken to avoid _________ and adsorption to surfaces.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Matching
One of the reasons the primary structure is important for a protein is that it determines the ______ the molecule adopts in aqueous solutions.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
One of the reasons the primary structure is important for a protein is that it determines the ______ the molecule adopts in aqueous solutions.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Matching
In order for DEAE to act as an anion exchanger, it must have a ______.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
In order for DEAE to act as an anion exchanger, it must have a ______.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Matching
In SDS-PAGE, disulfide-linked polypeptides can be separated after reacting the protein first with ______.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
In SDS-PAGE, disulfide-linked polypeptides can be separated after reacting the protein first with ______.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Matching
Molecules that contain a(n)______ are capable of absorbing light.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
Molecules that contain a(n)______ are capable of absorbing light.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
A fast and common method for determining the protein concentration in column effluent is
A)tandem mass spectrometry.
B)salting in with ammonium sulfate.
C)drying a portion and weighing the solid.
D)measuring light absorption at 280 nm.
E)Edman degradation.
A)tandem mass spectrometry.
B)salting in with ammonium sulfate.
C)drying a portion and weighing the solid.
D)measuring light absorption at 280 nm.
E)Edman degradation.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following has the most dramatic influence on the characteristics of an individual protein?
A)the amino-acid sequence
B)the amino-acid composition
C)the location of its encoding gene within the genome
D)the stereochemistry at the -carbon
E)the sequence of tRNA molecules involved in its translation
A)the amino-acid sequence
B)the amino-acid composition
C)the location of its encoding gene within the genome
D)the stereochemistry at the -carbon
E)the sequence of tRNA molecules involved in its translation

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Proteins are synthesized in vivo by the translation of
A)cDNA.
B)tRNA.
C)rRNA.
D)exons.
E)mRNA.
A)cDNA.
B)tRNA.
C)rRNA.
D)exons.
E)mRNA.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
The salting in of proteins can be explained by:
A)salt counter-ions reducing electrostatic attractions between protein molecules.
B)salt ions reducing the polarity of the solution.
C)salt ions increasing the hydrophobic interactions.
D)releasing hydrophobic proteins from nonpolar tissue environments.
E)hydration of the salt ions reducing solubility of proteins.
A)salt counter-ions reducing electrostatic attractions between protein molecules.
B)salt ions reducing the polarity of the solution.
C)salt ions increasing the hydrophobic interactions.
D)releasing hydrophobic proteins from nonpolar tissue environments.
E)hydration of the salt ions reducing solubility of proteins.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Matching
In ______ chromatography, a protein mixture must be applied to the column at a low pH so that the proteins will have a net positive charge and bind to the column.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
In ______ chromatography, a protein mixture must be applied to the column at a low pH so that the proteins will have a net positive charge and bind to the column.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Matching
If the cDNA for a protein has been cloned, it may be possible to obtain large quantities of the protein by _________________ in bacteria.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate
If the cDNA for a protein has been cloned, it may be possible to obtain large quantities of the protein by _________________ in bacteria.
A)electrophoresis
B)hydrophobic interaction
C)enzyme-linked immunosorbent assay
D)three-dimensional shape
E)N-terminal amino acid
F)negative charge
G)nucleases
H)chromophore
I)foaming
J)high level expression
K)2-mercaptoethanol
L)positive charge
M)cation exchange
N)pI
O)chymotrypsin
P)C-terminal amino acid
Q)Sodium dodecyl sulfate

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
You are trying to separate five proteins, which are listed below, by gel filtration chromatography.Which of the proteins will elute first from the column?
A)cytochrome c (12 kDa)
B)RNA polymerase (99 kDa)
C)glutamine synthetase (621 kDa)
D)interferon- (34 kDa)
E)hemoglobin (62 kDa)
A)cytochrome c (12 kDa)
B)RNA polymerase (99 kDa)
C)glutamine synthetase (621 kDa)
D)interferon- (34 kDa)
E)hemoglobin (62 kDa)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
You are purifying a nuclease by affinity chromatography.To determine which fractions contain the protein of interest, you test samples of all fractions for their ability to break down DNA.This is an example of
A)a binding assay.
B)a biological assay.
C)an enzyme assay.
D)an immunological assay.
E)none of the above
A)a binding assay.
B)a biological assay.
C)an enzyme assay.
D)an immunological assay.
E)none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following amino acids would be last to elute at pH 8.0 from an anion-exchange column?
A)lysine
B)alanine
C)glutamic acid
D)asparagine
E)glycine
A)lysine
B)alanine
C)glutamic acid
D)asparagine
E)glycine

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
A first step in purifying a protein that was initially associated with fatty substances would be
A)Coomassie Brilliant Blue dye staining.
B)analytical ultracentrifugation.
C)ELISA.
D)Western blotting.
E)hydrophobic interaction chromatography.
A)Coomassie Brilliant Blue dye staining.
B)analytical ultracentrifugation.
C)ELISA.
D)Western blotting.
E)hydrophobic interaction chromatography.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
A technique that can be used to separate proteins based primarily on their pI is called
A)ion-exchange chromatography.
B)gel filtration chromatography.
C)affinity chromatography.
D)isoelectric focusing.
E)hydrophobic interaction chromatography.
A)ion-exchange chromatography.
B)gel filtration chromatography.
C)affinity chromatography.
D)isoelectric focusing.
E)hydrophobic interaction chromatography.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
ELISA is an example of a(n):
A)enzyme assay.
B)biological assay.
C)binding assay.
D)immunological assay.
E)none of the above
A)enzyme assay.
B)biological assay.
C)binding assay.
D)immunological assay.
E)none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Which physical characteristic is not commonly used in protein separation?
A)solubility
B)stereochemistry
C)size
D)charge
E)polarity
A)solubility
B)stereochemistry
C)size
D)charge
E)polarity

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
The quantitation of proteins due to their absorbance at ~280 nm (UV region)is due to the large absorbtivity of the ________ amino acids.
A)anionic
B)dansylated
C)cleaved
D)polar
E)aromatic
A)anionic
B)dansylated
C)cleaved
D)polar
E)aromatic

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following amino acids would be first to elute at pH 8.0 from an anion-exchange column?
A)lysine
B)alanine
C)glutamic acid
D)asparagine
E)glycine
A)lysine
B)alanine
C)glutamic acid
D)asparagine
E)glycine

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Five graduate students prepare extracts from 5 different tissues.Each student measures the total amount of alcohol dehydrogenase and the total amount of protein in his or her extract.Which extract has the highest specific activity? 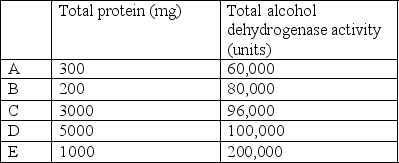


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
The acronym HPLC stands for
A)hydrophobic protein liquid chromatography.
B)high performance liquid chromatography.
C)hydrophilic partition liquid chromatography.
D)high priced liquid chromatography.
E)hydrostatic process liquid chromatography.
A)hydrophobic protein liquid chromatography.
B)high performance liquid chromatography.
C)hydrophilic partition liquid chromatography.
D)high priced liquid chromatography.
E)hydrostatic process liquid chromatography.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
The pK1, pK2, and pKR of the amino acid lysine are 2.2, 9.1, and 10.5, respectively.The pK1, pK2, and pKR of the amino acid arginine are 1.8, 9.0, and 12.5, respectively.A student at SDSU wants to use ion exchange chromatography to separate lysine from arginine.What pH is likely to work best for this separation?
A)1.5
B)2.5
C)5.5
D)7.5
E)10.5
A)1.5
B)2.5
C)5.5
D)7.5
E)10.5

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
What can be done to increase the rate at which a protein of interest moves down an ion-exchange chromatography column?
A)reduce the ion concentration in the eluant
B)add a small amount of a non-ionic detergents to the eluant
C)change the pH of the eluant
D)add a protease inhibitor to the eluant
E)reduce the temperature of the eluant
A)reduce the ion concentration in the eluant
B)add a small amount of a non-ionic detergents to the eluant
C)change the pH of the eluant
D)add a protease inhibitor to the eluant
E)reduce the temperature of the eluant

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
A technique that can be used to separate proteins based primarily on the presence of non-polar residues on their surface is called
A)ion-exchange chromatography.
B)gel filtration chromatography.
C)affinity chromatography.
D)gel electrophoresis.
E)hydrophobic interaction chromatography.
A)ion-exchange chromatography.
B)gel filtration chromatography.
C)affinity chromatography.
D)gel electrophoresis.
E)hydrophobic interaction chromatography.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
The pK1, pK2, and pKR of the amino acid histdine are 1.8, 9.3, and 6.0, respectively.The pK1, pK2, and pKR of the amino acid arginine are 1.8, 9.0, and 12.5, respectively.You have a mixture of histidine and arginine, how would you try to separate these two amino acids?
A)anion exchange chromatography at pH 2
B)anion exchange chromatography at pH 4
C)cation exchange chromatography at pH 2
D)cation exchange chromatography at pH 4
E)cation exchange chromatography at pH 9
A)anion exchange chromatography at pH 2
B)anion exchange chromatography at pH 4
C)cation exchange chromatography at pH 2
D)cation exchange chromatography at pH 4
E)cation exchange chromatography at pH 9

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following 'assays' would be most specific for a particular protein?
A)Bradford assay
B)UV absorptivity
C)radioimmunoassay
D)molar absorptivity
E)amino acid analysis
A)Bradford assay
B)UV absorptivity
C)radioimmunoassay
D)molar absorptivity
E)amino acid analysis

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
An enzyme-linked immunosorbent assay requires
A)a radioactive substrate.
B)a radioactive standard for binding to the antibody.
C)aromatic amino acids.
D)an antibody that binds the protein of interest.
E)a catalytic antibody.
A)a radioactive substrate.
B)a radioactive standard for binding to the antibody.
C)aromatic amino acids.
D)an antibody that binds the protein of interest.
E)a catalytic antibody.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Hydrophobic interaction chromatography can be used to separate proteins based on differences in
A)ionic charge.
B)solubility.
C)size.
D)polarity.
E)binding specificity.
A)ionic charge.
B)solubility.
C)size.
D)polarity.
E)binding specificity.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
A radioimmunoassay requires
A)an enzyme-linked antibody.
B)a coupled enzymatic reaction.
C)a radiolabeled antibody.
D)a catalytic antibody.
E)a radiolabeled standard protein that is used to compete for binding to the antibody.
A)an enzyme-linked antibody.
B)a coupled enzymatic reaction.
C)a radiolabeled antibody.
D)a catalytic antibody.
E)a radiolabeled standard protein that is used to compete for binding to the antibody.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
Adding additional salt to a protein solution can cause:
A)an increase in solubility called 'salting in'.
B)a decrease in solubility called 'salting out'.
C)protein precipitation from solution.
D)all of the above
E)none of the above
A)an increase in solubility called 'salting in'.
B)a decrease in solubility called 'salting out'.
C)protein precipitation from solution.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Disulfide bonds can be cleaved using
A)iodoacetate.
B)dansyl chloride.
C)2-mercaptoethanol ( -ME).
D)trypsin.
E)phenylisothiocyanate.
A)iodoacetate.
B)dansyl chloride.
C)2-mercaptoethanol ( -ME).
D)trypsin.
E)phenylisothiocyanate.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
The positive charge on proteins in electrospray ionization mass spectrometry is the result of
A)protons fired at the gas-phase protein molecules.
B)protonated side chains of Asp and Glu residues.
C)protonated side chains of Arg and Lys residues.
D)a high pH.
E)electrons fired at the gas-phase protein molecules.
A)protons fired at the gas-phase protein molecules.
B)protonated side chains of Asp and Glu residues.
C)protonated side chains of Arg and Lys residues.
D)a high pH.
E)electrons fired at the gas-phase protein molecules.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
A fast way for nature to generate new proteins is:
A)generation of pseudogenes.
B)mutation by neutral drift.
C)shuffling protein domains or motifs.
D)hypervariable positions.
E)liberal substitution.
A)generation of pseudogenes.
B)mutation by neutral drift.
C)shuffling protein domains or motifs.
D)hypervariable positions.
E)liberal substitution.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Although a protein's primary sequence can be inferred from the nucleotide sequence, modifications such as ______ can be determined most easily by tandem mass spectrometry followed by protein database searching.
A)phosphorylation
B)disulfide crosslinks
C)glycosylation
D)acetylation
E)all of the above
A)phosphorylation
B)disulfide crosslinks
C)glycosylation
D)acetylation
E)all of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
A protein that has had few changes in its amino acid sequence over evolutionary history is labeled
A)a fibrinopeptide.
B)evolutionarily conserved.
C)random.
D)a product of pseudogenes.
E)phylogenetic.
A)a fibrinopeptide.
B)evolutionarily conserved.
C)random.
D)a product of pseudogenes.
E)phylogenetic.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
______________ has emerged as a technique for protein sequencing.
A)NMR spectroscopy
B)Mass spectrometry
C)Gel electrophoresis
D)Phylogenetic analysis
E)Limited proteolysis
A)NMR spectroscopy
B)Mass spectrometry
C)Gel electrophoresis
D)Phylogenetic analysis
E)Limited proteolysis

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
SDS-PAGE separates proteins primarily due to differences in
A)isoelectric point.
B)mass.
C)polarity.
D)solubility.
E)amino acid sequence.
A)isoelectric point.
B)mass.
C)polarity.
D)solubility.
E)amino acid sequence.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Protein sequences are customarily 'reconstructed' from sequenced fragments because
A)protein purification invariably results in the fragmentation of the protein of interest.
B)proteins are naturally and inevitably cleaved by proteolytic enzymes.
C)proteins are composed of multiple subunits.
D)large polypeptides cannot be directly sequenced.
E)all of the above
A)protein purification invariably results in the fragmentation of the protein of interest.
B)proteins are naturally and inevitably cleaved by proteolytic enzymes.
C)proteins are composed of multiple subunits.
D)large polypeptides cannot be directly sequenced.
E)all of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
A phylogenetic tree depicts ___________ of proteins.
A)folding patterns
B)hypervariable residues
C)invariable residues
D)evolutionary relationships
E)gene sequences
A)folding patterns
B)hypervariable residues
C)invariable residues
D)evolutionary relationships
E)gene sequences

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Which of these reagents is commonly used to determine the number of polypeptides in a protein?
A)iodoacetate
B)dansyl chloride
C)2-mercaptoethanol ( -ME)
D)cyanogen bromide
E)DEAE
A)iodoacetate
B)dansyl chloride
C)2-mercaptoethanol ( -ME)
D)cyanogen bromide
E)DEAE

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following substances cannot be used to cleave peptide bonds in polypeptides?
A)trypsin
B)cyanogen bromide
C)endopeptidases
D)2-mercaptoethanol
E)pepsin
A)trypsin
B)cyanogen bromide
C)endopeptidases
D)2-mercaptoethanol
E)pepsin

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Enzymes that hydrolyze the internal peptide bonds (not the peptide bonds of the terminal amino acids)of a protein are classified as
A)oxidoreductases.
B)lyases.
C)endopeptidases.
D)nucleases.
E)exopeptidases.
A)oxidoreductases.
B)lyases.
C)endopeptidases.
D)nucleases.
E)exopeptidases.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Paralogous genes are
A)genes that do not encode protein.
B)genes of slowly evolving proteins.
C)relics of genes that are not expressed.
D)genes of rapidly evolving proteins.
E)the results of gene duplication.
A)genes that do not encode protein.
B)genes of slowly evolving proteins.
C)relics of genes that are not expressed.
D)genes of rapidly evolving proteins.
E)the results of gene duplication.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Edman degradation can be used to
A)identify the N-terminal amino acid of a polypeptide.
B)identify the C-terminal amino acid of a polypeptide.
C)separate the subunits of a multi-subunit protein.
D)cleave a protein at specific sites.
E)cleave disulfide bonds within a protein so that the individual polypeptides can be separated.
A)identify the N-terminal amino acid of a polypeptide.
B)identify the C-terminal amino acid of a polypeptide.
C)separate the subunits of a multi-subunit protein.
D)cleave a protein at specific sites.
E)cleave disulfide bonds within a protein so that the individual polypeptides can be separated.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
The peptide Leu─Cys─Arg─Ser─Gln─Met is subjected to Edman degradation.In the first cycle the peptide first reacts with phenylisothiocyanate under basic conditions.The product of this reaction is incubated with anhydrous trifluoroacetic acid and subsequently with an aqueous acid.What are the products generated in the first cycle.
A)PTH─Leu, PTH─Cys, PTH─Arg, PTH─Ser, PTH─Gln, and PTH─Met
B)PTH─Leu─Cys─Arg─Ser─Gln─Met
C)PTH─Met and Leu─Cys─Arg─Ser─Gln─Met
D)PTH─Leu─Cys and PTH─Arg─Ser─Gln─Met
E)PTH─Leu and Cys─Arg─Ser─Gln─Met
A)PTH─Leu, PTH─Cys, PTH─Arg, PTH─Ser, PTH─Gln, and PTH─Met
B)PTH─Leu─Cys─Arg─Ser─Gln─Met
C)PTH─Met and Leu─Cys─Arg─Ser─Gln─Met
D)PTH─Leu─Cys and PTH─Arg─Ser─Gln─Met
E)PTH─Leu and Cys─Arg─Ser─Gln─Met

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
In two homologous proteins, which residue is most likely to replace a Glu residue as a conservative substitution?
A)Asp
B)Trp
C)Met
D)Ile
E)Lys
A)Asp
B)Trp
C)Met
D)Ile
E)Lys

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
___________ is an example of a very slowly evolving protein.
A)Histone H4
B)Hemoglobin
C)Cytochrome c
D)Fibrinopeptides
E)none of the above
A)Histone H4
B)Hemoglobin
C)Cytochrome c
D)Fibrinopeptides
E)none of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these techniques uses antibodies to detect very small amounts of specific proteins following separation by SDS-PAGE.
A)immunoblotting
B)silverstaining
C)Coomassie Brilliant Blue staining
D)ELISA
E)RIA
A)immunoblotting
B)silverstaining
C)Coomassie Brilliant Blue staining
D)ELISA
E)RIA

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Which of these are commonly used to cleave peptide bonds in polypeptides?
A)2-mercaptoethanol ( -ME)
B)dansyl chloride
C)iodoacetate
D)sodium dodecyl sulfate
E)trypsin
A)2-mercaptoethanol ( -ME)
B)dansyl chloride
C)iodoacetate
D)sodium dodecyl sulfate
E)trypsin

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these techniques is used to separate proteins mainly based on mass?
A)polyacrylamide gel electrophoresis (in the absence of SDS)
B)SDS-PAGE
C)isoelectric focusing
D)immunoblotting
E)Western blotting
A)polyacrylamide gel electrophoresis (in the absence of SDS)
B)SDS-PAGE
C)isoelectric focusing
D)immunoblotting
E)Western blotting

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
We are able to purify proteins because they differ from each other in various physical or chemical properties.List 5 physicochemical properties of proteins that can be used as basis for their separation.Give a method of separation based on each of these properties (match the method with the property).

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Proteins can vary in size from approximately 40 to 34,000 amino acids.
a.Why is there a lower limit to the size of proteins?
b.Why is there an upper limit to the size of polypeptides?
a.Why is there a lower limit to the size of proteins?
b.Why is there an upper limit to the size of polypeptides?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
One technique commonly used in protein purification is chromatography.
a.Explain briefly the general principle of column chromatography
b.Name four types of chromatography and indicate for each of these types the basis for separation (match types of chromatography with the properties that form the basis for separation).
a.Explain briefly the general principle of column chromatography
b.Name four types of chromatography and indicate for each of these types the basis for separation (match types of chromatography with the properties that form the basis for separation).

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Proteins are often constructed from multiple segments of 40-200 amino acid residues, commonly called
A)pseudogenes.
B)hypervariable residues.
C)protolytic fragments.
D)domains.
E)subunits.
A)pseudogenes.
B)hypervariable residues.
C)protolytic fragments.
D)domains.
E)subunits.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
You have purified the receptor for a hormone by affinity chromatography.During gel filtration chromatography under native conditions the receptor elutes between pyruvate decarboxylase (250 kDa)and glutamine synthetase (620 kDa).During SDS-PAGE, in the absence of reducing agents, the receptor migrates as a single band of approximately 230 kDa.When SDS-PAGE is carried out in the presence of 2-mercaptoethanol the receptor migrates as two bands of approximately 95 and 135 kDa.Explain this result.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Ion exchange chromatography is a commonly used method for separation of biomolecules.
a.What are the two types of ion exchange chromatography?
b.In what order would Arg, Val, and Glu elute from a carboxymethyl column at pH 6.0.Carboxymethyl is negatively charged at pH 6.0.
c.You are trying to purify a protein using ion exchange chromatography.Unfortunately, your protein remains bound to the ion exchange column or it is eluting very slowly.What are the two changes you can make, to try to elute your protein more quickly from this column?
a.What are the two types of ion exchange chromatography?
b.In what order would Arg, Val, and Glu elute from a carboxymethyl column at pH 6.0.Carboxymethyl is negatively charged at pH 6.0.
c.You are trying to purify a protein using ion exchange chromatography.Unfortunately, your protein remains bound to the ion exchange column or it is eluting very slowly.What are the two changes you can make, to try to elute your protein more quickly from this column?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
A variety of chromatographic techniques are available for protein purification.
a.Explain briefly the principle of hydrophobic interaction chromatography.
b.Name three changes that can be made to the eluant that can be used to speed up elution of the protein of interest from a hydrophobic interaction chromatography column.
a.Explain briefly the principle of hydrophobic interaction chromatography.
b.Name three changes that can be made to the eluant that can be used to speed up elution of the protein of interest from a hydrophobic interaction chromatography column.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
You are interested in receptor protein-tyrosine kinases and you are purifying a novel phosphotyrosine-binding protein (NPBP-1).In order to characterize this protein you need to separate it from three other proteins (X, Y, and Z)that are still present in your partially purified material.The proteins in your preparation have the following properties:
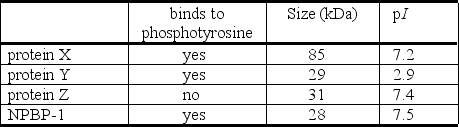 a.What type of separation technique can be used to separate protein NPBP-1 from protein X?
a.What type of separation technique can be used to separate protein NPBP-1 from protein X?
b.What type of separation technique can be used to separate protein NPBP-1 from protein Y?
c.What type of separation technique can be used to separate protein NPBP-1 from protein Z?
 a.What type of separation technique can be used to separate protein NPBP-1 from protein X?
a.What type of separation technique can be used to separate protein NPBP-1 from protein X?b.What type of separation technique can be used to separate protein NPBP-1 from protein Y?
c.What type of separation technique can be used to separate protein NPBP-1 from protein Z?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


