Deck 6: Proteins: Three-Dimensional Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 6: Proteins: Three-Dimensional Structure
1
Matching
The strength of _______ comes from close packing of glycine residues and the characteristics of hydroxyproline allowing formation of a left- handed helical conformation which combines with two other left handed structures to form a right-handed triplet helix.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
The strength of _______ comes from close packing of glycine residues and the characteristics of hydroxyproline allowing formation of a left- handed helical conformation which combines with two other left handed structures to form a right-handed triplet helix.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
collagen
2
Matching
________ is a fibrous protein that contains a hydrophobic amino acid approximately every 4 residues which forms an α helix with one hydrophobic side.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
________ is a fibrous protein that contains a hydrophobic amino acid approximately every 4 residues which forms an α helix with one hydrophobic side.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
keratin
3
Matching
The _____________ of an amino acid can be used to predict whether an amino acid side chain folds towards the inside or outside of a globular protein.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
The _____________ of an amino acid can be used to predict whether an amino acid side chain folds towards the inside or outside of a globular protein.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
hydropathy
4
In a Ramachandran diagram, a larger area represents sterically allowed torsion angles of and that are allowed in _____ rather than in ______ because there is greater opportunity for separation of amino acid side chains.
A)secondary structure…tertiary structure
B)α helix…β sheet
C)β sheet…α helix
D)tertiary structure…secondary structure
E)none of the above
A)secondary structure…tertiary structure
B)α helix…β sheet
C)β sheet…α helix
D)tertiary structure…secondary structure
E)none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is true regarding crystalline proteins?
A)Many crystallized enzyme proteins remain catalytically active.
B)The diffractive pattern observed during X-ray exposure to the crystal can be used to calculate the electron density map of the crystalline protein.
C)The larger region indicating electron density with in the electron density map, the more accurate the structure determination.
D)A and B are true.
E)A, B, and C are true.
A)Many crystallized enzyme proteins remain catalytically active.
B)The diffractive pattern observed during X-ray exposure to the crystal can be used to calculate the electron density map of the crystalline protein.
C)The larger region indicating electron density with in the electron density map, the more accurate the structure determination.
D)A and B are true.
E)A, B, and C are true.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following changes would not alter the functional characteristics of α keratin?
A)Increasing the number of residues per turn to 4.1 while maintaining the same amino acid sequence.
B)Substitution of a hydrophilic amino acid for a hydrophobic amino acid at position a and d of the 7-residue pseudorepeat.
C)Decreasing the number of cysteine amino acids within each protofilament.
D)Changing the environment surrounding the protein to one that is more reductive.
E)All of the above would alter the functional characteristics of keratin.
A)Increasing the number of residues per turn to 4.1 while maintaining the same amino acid sequence.
B)Substitution of a hydrophilic amino acid for a hydrophobic amino acid at position a and d of the 7-residue pseudorepeat.
C)Decreasing the number of cysteine amino acids within each protofilament.
D)Changing the environment surrounding the protein to one that is more reductive.
E)All of the above would alter the functional characteristics of keratin.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Matching
The overall arrangement of the regular structural elements such as the α helix and the β sheet in the protein are considered the protein's ______.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
The overall arrangement of the regular structural elements such as the α helix and the β sheet in the protein are considered the protein's ______.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following has (have)both a favorable hydrogen bonding pattern and and values that fall within the allowed Ramachandran conformational regions?
A)( helix)
B)collagen helix
C)( sheet)
D)all of the above
E)none of the above
A)( helix)
B)collagen helix
C)( sheet)
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Matching
-Repeating values of and make up predictable orientations of amino acid with a chain, this predictable orientation forms___________.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
-Repeating values of and make up predictable orientations of amino acid with a chain, this predictable orientation forms___________.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of these characteristics is not true for the helix?
A)There are 3.6 amino acids per turn.
B)There is a requirement for glycine every third amino acid residue.
C)A hydrogen bond forms between the carbonyl oxygen of the nth amino acid residue and the - NH group of the (n + 4)th amino acid residue.
D)Proline is typically not found in the helix.
E)It is right-handed.
A)There are 3.6 amino acids per turn.
B)There is a requirement for glycine every third amino acid residue.
C)A hydrogen bond forms between the carbonyl oxygen of the nth amino acid residue and the - NH group of the (n + 4)th amino acid residue.
D)Proline is typically not found in the helix.
E)It is right-handed.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Matching
In vivo protein folding is often is assisted by ______.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
In vivo protein folding is often is assisted by ______.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Matching
-In a ______, the and angles of the peptide backbone would orient atoms closer than their van der Waals distance.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
-In a ______, the and angles of the peptide backbone would orient atoms closer than their van der Waals distance.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Matching
In most peptide groups the ______ conformation is not sterically favored.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
In most peptide groups the ______ conformation is not sterically favored.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following gives the best example of a nonrepetitive structure in a protein?
A)a random sequence of 12 amino acids with high Pα values forming an α helix
B)an amino acid sequence with the following pattern "…a-b-c-d-e-a-b-c-d-a-b-c-d…"
C)a 13 residue α helix with a Gln at position n+12 which hydrogen bonds to a residue at position n+10
D)All of the above statements describe nonrepetitive protein structures.
E)None of the above describe nonrepetitive protein structures.
A)a random sequence of 12 amino acids with high Pα values forming an α helix
B)an amino acid sequence with the following pattern "…a-b-c-d-e-a-b-c-d-a-b-c-d…"
C)a 13 residue α helix with a Gln at position n+12 which hydrogen bonds to a residue at position n+10
D)All of the above statements describe nonrepetitive protein structures.
E)None of the above describe nonrepetitive protein structures.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements is true regarding collagen?
A)The inability to hydroxylate proline results in the inability to synthesize collagen.
B)The α helical structure is ideal for intertwining 3 filaments.
C)Hydrogen bonds between the ─OH groups of Hyp residues stabilize the helix.
D)The requirement for glycine every 3rd amino acid is essential for the triplet helix formation.
E)On average, there is one proline for every hydroxyproline.
A)The inability to hydroxylate proline results in the inability to synthesize collagen.
B)The α helical structure is ideal for intertwining 3 filaments.
C)Hydrogen bonds between the ─OH groups of Hyp residues stabilize the helix.
D)The requirement for glycine every 3rd amino acid is essential for the triplet helix formation.
E)On average, there is one proline for every hydroxyproline.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Matching
A historical experiment exploring denaturation upon β- mercaptoethanol reduction of disulfide bonds and spontaneous renaturation upon dialysis to remove the β- mercaptoethanol was carried out using the protein ______.This experiment demonstrated the importance of disulfide bonds and amino acid sequence in folding of proteins.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
A historical experiment exploring denaturation upon β- mercaptoethanol reduction of disulfide bonds and spontaneous renaturation upon dialysis to remove the β- mercaptoethanol was carried out using the protein ______.This experiment demonstrated the importance of disulfide bonds and amino acid sequence in folding of proteins.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
In a protein, the most conformationally restricted amino acid is ______; the least conformationally restricted is ______.
A)Trp, Gly
B)Met, Cys
C)Pro, Gly
D)Ile, Ala
E)Ala, Pro
A)Trp, Gly
B)Met, Cys
C)Pro, Gly
D)Ile, Ala
E)Ala, Pro

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Which of these characteristics does not describe the sheet?
A)Amino acid side chains are located both above and below the sheet.
B)( sheets have a pleated edge-on appearance.)
C)They can exist in either parallel or antiparallel configurations.
D)The sheets contain as few as two and as many as 22 polypeptide chains.
E)Parallel sheets containing fewer than five chains are the most common.
A)Amino acid side chains are located both above and below the sheet.
B)( sheets have a pleated edge-on appearance.)
C)They can exist in either parallel or antiparallel configurations.
D)The sheets contain as few as two and as many as 22 polypeptide chains.
E)Parallel sheets containing fewer than five chains are the most common.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement below does not describe fibrous proteins?
A)Domains have a globular fold.
B)These proteins usually contain only one type of secondary structure.
C)These proteins usually exhibit structural or protective characteristics.
D)These proteins have usually elongated hydrophilic surfaces.
E)These proteins are usually insoluble in water.
A)Domains have a globular fold.
B)These proteins usually contain only one type of secondary structure.
C)These proteins usually exhibit structural or protective characteristics.
D)These proteins have usually elongated hydrophilic surfaces.
E)These proteins are usually insoluble in water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Matching
A rigid, planar structure between at least two amino acids consisting of about 40% double bond character is characteristic of a ______.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha
A rigid, planar structure between at least two amino acids consisting of about 40% double bond character is characteristic of a ______.
A)tertiary structure
B)keratin
C)molecular chaperones
D)hydropathy
E)cis
F)trans
G)sterically forbidden conformation
H)regular secondary structure
I)collagen
J)peptide bond
K)ribonuclease A (RNase A)
L)alpha

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following amino acids combinations have side chains with groups that have the greatest ability to stabilize the tertiary structure of a protein?
A)Lys and Arg
B)Cys and Glu
C)Glu and Lys
D)Gln and Glu
E)Pro and Asp
A)Lys and Arg
B)Cys and Glu
C)Glu and Lys
D)Gln and Glu
E)Pro and Asp

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
When comparing similarities among multiple protein structures, which of the following is false?
A)Proteins with the same function from a different species are likely to have similar motifs.
B)Proteins with the same function from different species are likely to be more similar in sequence than in structure.
C)An effective protein motif isl likely be observed in multiple proteins.
D)Proteins with the same motifs are likely to perform similar functions.
E)None of the above statements are false.
A)Proteins with the same function from a different species are likely to have similar motifs.
B)Proteins with the same function from different species are likely to be more similar in sequence than in structure.
C)An effective protein motif isl likely be observed in multiple proteins.
D)Proteins with the same motifs are likely to perform similar functions.
E)None of the above statements are false.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
The structure of hen egg white protein has been solved and the torsion angles and are shown for each residue in the table below.What structure motif most likely forms as a result of this protein sequence? 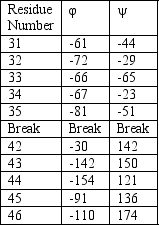
A)β strand connected to another β strand with a break (or loop/turn)in between
B)α Helix connect to another α Helix with a break (or loop/turn)in between
C)β strand connected to another β strand with a alpha helix in between
D)α Helix connected to a β strand with a break (or loop/turn)in between
E)None of the above are correct.

A)β strand connected to another β strand with a break (or loop/turn)in between
B)α Helix connect to another α Helix with a break (or loop/turn)in between
C)β strand connected to another β strand with a alpha helix in between
D)α Helix connected to a β strand with a break (or loop/turn)in between
E)None of the above are correct.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Evolutionary processes have
A)increased the stability of 4° structures.
B)decreased the number of subunits.
C)increased similarity amount 1° structures.
D)enhanced efficient folding pathways.
E)all of the above
A)increased the stability of 4° structures.
B)decreased the number of subunits.
C)increased similarity amount 1° structures.
D)enhanced efficient folding pathways.
E)all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
For -sheets, the terms 'parallel' and 'antiparalllel' refer to ___________.
A)the 'direction' of the associated peptide strands
B)the orientation of the amide cross-links
C)the quaternary structure of the protein
D)the orientation of the hydrogen bonding
E)the topology of the reverse turns
A)the 'direction' of the associated peptide strands
B)the orientation of the amide cross-links
C)the quaternary structure of the protein
D)the orientation of the hydrogen bonding
E)the topology of the reverse turns

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
The first step in the folding of disordered polypeptides into ordered functional protein is the formation of ______.
A)1o structure
B)2° structure
C)3° structure
D)4° structure
E)hydrogen bonds
A)1o structure
B)2° structure
C)3° structure
D)4° structure
E)hydrogen bonds

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would be most stable based on the information you have learned about protein structure?
A)a loop region with 8 amino acids
B)a β sheet region made up of amino acids Val, Ile, Phe
C)an α helix made up of Cys, Pro, and Phe
D)a β hairpin with 12 amino acids
E)All have equal stability.
A)a loop region with 8 amino acids
B)a β sheet region made up of amino acids Val, Ile, Phe
C)an α helix made up of Cys, Pro, and Phe
D)a β hairpin with 12 amino acids
E)All have equal stability.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
The Protein Data Bank (PDB)is a database provides structural information about proteins that may be useful for which of the following?
I.A researcher studying the changes in protein fold associated with prions.
II.A researcher classifying structural elements by function.
III A researcher designing a compound to bind tightly to a particular region in the protein.
A)I only
B)II only
C)III only
D)I, II
E)I, II, II
I.A researcher studying the changes in protein fold associated with prions.
II.A researcher classifying structural elements by function.
III A researcher designing a compound to bind tightly to a particular region in the protein.
A)I only
B)II only
C)III only
D)I, II
E)I, II, II

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Imagine that a researcher treated a protein with a high concentration of a chaotropic agent.Which of the following is the most likely result of the treatment?
I.Nonpolar portions of the protein become more soluble.
II.The protein begins to denature ,
III.The protein stability increases due to hydrophobic collapse,
A)I, II, III
B)I, II
C)II, III
D)I, III
E)II
I.Nonpolar portions of the protein become more soluble.
II.The protein begins to denature ,
III.The protein stability increases due to hydrophobic collapse,
A)I, II, III
B)I, II
C)II, III
D)I, III
E)II

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Protein diseases can be caused by which of the following
A)mutations affecting the 1° structure.
B)mutations affecting the 3° structure.
C)changes in the post-synthetic processing of proteins.
D)All of the above are potential causes.
E)None of the above are potential causes.
A)mutations affecting the 1° structure.
B)mutations affecting the 3° structure.
C)changes in the post-synthetic processing of proteins.
D)All of the above are potential causes.
E)None of the above are potential causes.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Conventional one dimensional NMR spectroscopy is not generally an effective tool for determination of protein structure because…
I.Proteins (including small proteins)have a high number of hydrogen atoms.
II.NMR requires a high quality protein crystal.
III.The NMR spectra exhibit high peak overlap.
A)I and II
B)II and III
C)I and III
D)I, II, and III
E)III only
I.Proteins (including small proteins)have a high number of hydrogen atoms.
II.NMR requires a high quality protein crystal.
III.The NMR spectra exhibit high peak overlap.
A)I and II
B)II and III
C)I and III
D)I, II, and III
E)III only

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
In general molecular chaperone proteins function by
A)mediating disulfide bond formation
B)synthesizing new proteins when one is misfolded.
C)preventing premature folding by binding hydrophobic regions of the protein.
D)enhancing salt bridge formation.
E)none of the above
A)mediating disulfide bond formation
B)synthesizing new proteins when one is misfolded.
C)preventing premature folding by binding hydrophobic regions of the protein.
D)enhancing salt bridge formation.
E)none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
The classic experiment demonstrating that reduced and denatured RNase A could refold into the native form demonstrates that _______.
A)1° structure can determine 3° structure
B)denaturation does not disrupt protein 2° structure
C)disulfide bonds do not stabilize folded proteins
D)All of the above.
E)None of the above
A)1° structure can determine 3° structure
B)denaturation does not disrupt protein 2° structure
C)disulfide bonds do not stabilize folded proteins
D)All of the above.
E)None of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
Chaperonins such as the GroEL/ES system
A)function with thermophilic proteins only.
B)are required at low pH.
C)require ATP hydrolysis.
D)in vitro only.
E)function in a nonaqueous environment.
A)function with thermophilic proteins only.
B)are required at low pH.
C)require ATP hydrolysis.
D)in vitro only.
E)function in a nonaqueous environment.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
The low pH found in the gut can enhance the digestibility of dietary protein by causing ___.
A)amide hydrolysis
B)protein denaturation
C)disulfide reduction
D)prion formation
E)cysteine oxidation
A)amide hydrolysis
B)protein denaturation
C)disulfide reduction
D)prion formation
E)cysteine oxidation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Noncovalent forces that stabilize protein structure include all of the following except __________.
A)the hydrophobic effect
B)salt bridges
C)electrostatic interactions with metal ions
D)hydrogen bonding
E)disulfide bridges
A)the hydrophobic effect
B)salt bridges
C)electrostatic interactions with metal ions
D)hydrogen bonding
E)disulfide bridges

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Of the following, which amino acid is most likely to be found in position 1 or 4 on α keratin?
A)Phe
B)Ala
C)Lys
D)Trp
E)Pro
A)Phe
B)Ala
C)Lys
D)Trp
E)Pro

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
The structure and sequence of a protein of unknown function was examined.Which of the following provides the best prediction of the protein's function?
A)the observation of several disordered α helical domains.
B)the observation of multiple protein subunits.
C)the observation of motif known as the Rossmann fold.
D)the observation of a large number of random coil regions.
E)All of the above offer excellent prediction of the protein's function.
A)the observation of several disordered α helical domains.
B)the observation of multiple protein subunits.
C)the observation of motif known as the Rossmann fold.
D)the observation of a large number of random coil regions.
E)All of the above offer excellent prediction of the protein's function.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following occurs first when folding a disordered polypeptide chain into a stable protein formation?
A)formation of a low energy state
B)association of ordered subunits
C)aggregation of hydrophobic regions in the protein
D)tertiary structure refinement
E)formation of a low entropy state
A)formation of a low energy state
B)association of ordered subunits
C)aggregation of hydrophobic regions in the protein
D)tertiary structure refinement
E)formation of a low entropy state

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
In the absence of ascorbic acid, prolyl oxidase is unable to oxidize proline residues in collagen to hydroxyproline, resulting in:
A)lathyrism
B)prion diseases
C)amyloid formation
D)scurvy
E)allysine
A)lathyrism
B)prion diseases
C)amyloid formation
D)scurvy
E)allysine

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
When considering fibrous proteins, which of the following statements is TRUE?
A)Noncovalent interactions contribute to the strength of all of these proteins.
B)All of them consist of helix structure.
C)All of them require vitamin C.
D)Decrease in amounts of any of them cause scurvy.
E)All of these are true of fibrous proteins.
A)Noncovalent interactions contribute to the strength of all of these proteins.
B)All of them consist of helix structure.
C)All of them require vitamin C.
D)Decrease in amounts of any of them cause scurvy.
E)All of these are true of fibrous proteins.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Based on what you know about fibrous protein structure and sequence, what type of fibrous protein is this sequence most likely to from (You can assume that the protein is longer than what is shown and is repeating as shown, also note the polarity of each amino acid.)?
Val - Cys - Lys - Val - Cys - Ala - Cys - Val - Cys - Lys - Val - Cys - Ala - Cys
A)( keratin)
B)β keratin
C)collagen
D)pleated collagen
E)This sequence cannot be from any of the structural proteins.
Val - Cys - Lys - Val - Cys - Ala - Cys - Val - Cys - Lys - Val - Cys - Ala - Cys
A)( keratin)
B)β keratin
C)collagen
D)pleated collagen
E)This sequence cannot be from any of the structural proteins.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the characteristics of collagen structure listed below contrubute to the tensi le strength of collagen?
I.Collagen is made up of a triplet helix where 3 left-handed helices twist together in a right handed sense.
II.Collagen includes at repeating sequence of amino acids with glycine every 3 amino acids in a helix with about 3 amino acids per turn.
III.The three left-handed helices are staggered to allow close packing between glycine residues and rigidity from the bulky and inflexible proline/hydroxyproline.
A)I
B)I and II
C)I, II, and III
D)II and III
E)I and III
I.Collagen is made up of a triplet helix where 3 left-handed helices twist together in a right handed sense.
II.Collagen includes at repeating sequence of amino acids with glycine every 3 amino acids in a helix with about 3 amino acids per turn.
III.The three left-handed helices are staggered to allow close packing between glycine residues and rigidity from the bulky and inflexible proline/hydroxyproline.
A)I
B)I and II
C)I, II, and III
D)II and III
E)I and III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
When solving a protein structure using X-ray crystallography, the crystallographer generates a 3-D grid called an electron density map based on the observed diffraction pattern.The higher the resolution, the more detailed the electron density map and therefore the easier it is to identify what atoms (and therefore what amino acids)are in a given position.Based on the three choices below, in which of the following groups could the two of amino acids be the easiest to differentiate regardless of resolution?
I.Leucine vs.Isoleucine
II.Phenylalanine vs.Alanine
III.Glutamate vs.Glutamic acid
A)Those in both groups I and II could be differentiated
B)Those in both groups I and III could be differentiated
C)Only those in group II could be differentiated
D)Only those in group III could be differentiated
E)Only those in groupI could be differentiated
I.Leucine vs.Isoleucine
II.Phenylalanine vs.Alanine
III.Glutamate vs.Glutamic acid
A)Those in both groups I and II could be differentiated
B)Those in both groups I and III could be differentiated
C)Only those in group II could be differentiated
D)Only those in group III could be differentiated
E)Only those in groupI could be differentiated

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Hydrogen bonds and maximum separation of amino acid side chains make the _____very stable and energetically ______________.
A)α helix and β sheet, favorable
B)α helix, unfavorable
C)β sheet, unfavorable
D)α helix, favorable
E)β sheet, favorable
A)α helix and β sheet, favorable
B)α helix, unfavorable
C)β sheet, unfavorable
D)α helix, favorable
E)β sheet, favorable

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Examine the three sequences below for collagen-like proteins.If hydrogen bonding were the most important feature in determining strength in fibrous proteins, which of the following sequences likely has the highest melting temperature and why? (Note: Flp = fluoroproline; Hyp = hydroxyproline)
I.Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly
II.Pro-Flp-Gly-Pro-Flp-Gly-Pro-Flp-Gly
III.Gly-Pro-Thr-Gly-Pro-Thr-Gly-Pro-Thr
A)"1" because Hyp has OH groups
B)"1" because the electronegativity of oxygen is greater
C)"2" because the electronegativity of proline is greater
D)"2" because the electronegativity of the -OH group increases hydrogen bond strength
E)"3" because Thr is a small amino acid which allows close packing
I.Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly
II.Pro-Flp-Gly-Pro-Flp-Gly-Pro-Flp-Gly
III.Gly-Pro-Thr-Gly-Pro-Thr-Gly-Pro-Thr
A)"1" because Hyp has OH groups
B)"1" because the electronegativity of oxygen is greater
C)"2" because the electronegativity of proline is greater
D)"2" because the electronegativity of the -OH group increases hydrogen bond strength
E)"3" because Thr is a small amino acid which allows close packing

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Noncovalent interactions account for the strength of which of the following structural proteins?
A)( keratin)
B)collagen
C)pleated collagen
D)A and B
E)B and C
A)( keratin)
B)collagen
C)pleated collagen
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
A helix has hydrogen bonds between the carbonyl group from residue "n" and the amino group of residue "n+6," which of the following is TRUE?
A)It has 3.6 residues per turn.
B)It is a random coil, not a helix.
C)It is an α helix.
D)It has more residues per turn than an α helix.
E)It has fewer residues per turn than an α helix.
A)It has 3.6 residues per turn.
B)It is a random coil, not a helix.
C)It is an α helix.
D)It has more residues per turn than an α helix.
E)It has fewer residues per turn than an α helix.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following contribute to the minimization of energy that occurs with protein folding?
A)orientating amino acid groups to maximize hydrogen bonding
B)folding hydrophobic groups towards the exterior of the protein
C)burying polar groups towards the interior of the protein
D)extensive cavity formation
E)all of the above
A)orientating amino acid groups to maximize hydrogen bonding
B)folding hydrophobic groups towards the exterior of the protein
C)burying polar groups towards the interior of the protein
D)extensive cavity formation
E)all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following structural proteins has the greatest elasticity?
A)( keratin)
B)β ketatin
C)collagen
D)pleated collagen
E)A and B are equal
A)( keratin)
B)β ketatin
C)collagen
D)pleated collagen
E)A and B are equal

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
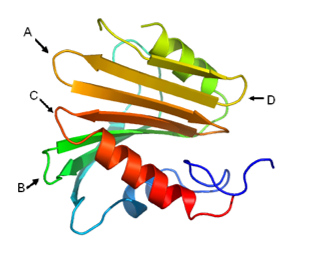
Which of the following lines in the figure at right indicates a hairpin structure?
A)A, C, D
B)B and C
C)A only
D)A, B, and D
E)C only

A)A, C, D
B)B and C
C)A only
D)A, B, and D
E)C only

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following best describes the cause of Creutzfeld-Jakob Disease (a disease which human can develop with symptoms similar to those of mad cow disease)?
A)aggregation of a misfolded protein.
B)aggregation of random coil regions on a protein.
C)ingestion of ammonium salts.
D)the serious side effects of experimental treatment with Quinacrine
E)All are potential causes Creutzfeld-Jakob disease.
A)aggregation of a misfolded protein.
B)aggregation of random coil regions on a protein.
C)ingestion of ammonium salts.
D)the serious side effects of experimental treatment with Quinacrine
E)All are potential causes Creutzfeld-Jakob disease.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Proteins can denature due to a change in
A)pH.
B)temperature.
C)ionic strength.
D)all of the above
E)none of the above
A)pH.
B)temperature.
C)ionic strength.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
In a Ramachandran diagram the region representing the angles of and that correspond to those commonly made by an amino acid that favors a left-handed helix are different from those angles commonly made by an amino acid that favors right-handed helix formation.Which of the following statements provides a plausible explanation for this difference?
A)Groups which would normally undergo high steric hindrance in the right-handed arrangement are separated maximally in the left-handed arrangement.
B)Left-handed helices have smaller pitch than right-handed helices.
C)The peptide backbone can coil tighter in the left-handed helices than in the right-handed helices.
D)Left-handed helices exhibit cyclic symmetry, while righthanded helices are asymmetric.
E)All of the above are plausible explanations.
A)Groups which would normally undergo high steric hindrance in the right-handed arrangement are separated maximally in the left-handed arrangement.
B)Left-handed helices have smaller pitch than right-handed helices.
C)The peptide backbone can coil tighter in the left-handed helices than in the right-handed helices.
D)Left-handed helices exhibit cyclic symmetry, while righthanded helices are asymmetric.
E)All of the above are plausible explanations.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
Protein dynamics is a field of study that examines the movements with in a protein.Which type of protein structure determination would be most useful to study this type of change?
A)X-ray crystallography
B)Nuclear Magnetic Resonance (NMR)
C)X-ray absorption spectroscopy
D)A and B
E)B and C
A)X-ray crystallography
B)Nuclear Magnetic Resonance (NMR)
C)X-ray absorption spectroscopy
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
A chaperonin
A)helps fold some proteins in their lowest energy state.
B)is required for all proteins to fold properly.
C)mediates the unfolding of proteins.
D)is required for protein denaturation.
E)counteracts the laws of thermodynamics.
A)helps fold some proteins in their lowest energy state.
B)is required for all proteins to fold properly.
C)mediates the unfolding of proteins.
D)is required for protein denaturation.
E)counteracts the laws of thermodynamics.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Native protein purifications often require multiple reaction steps in order to purify the protein of interest from other proteins.One method used for protein separation in purification procedures is a change from water to an organic solvent.Which of the following would be accomplished by this solvent change?
A)Proteins with hydrophobic groups on the interior would maintain their native state.
B)Proteins with hydrophilic groups on the exterior would denature and likely precipitate.
C)Proteins with exposed hydrophobic groups would maintain their structure and remain in solution.
D)Both A and B would occur.
E)Both B and C would occur.
A)Proteins with hydrophobic groups on the interior would maintain their native state.
B)Proteins with hydrophilic groups on the exterior would denature and likely precipitate.
C)Proteins with exposed hydrophobic groups would maintain their structure and remain in solution.
D)Both A and B would occur.
E)Both B and C would occur.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Examine the three sequences below for collagen-like proteins and their melting temperatures (Tm).(Note: Flp = fluoroproline; Hyp = hydroxyproline)Based on this data, what is the most important feature in determining the strength of the collagen protein?
1)…-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-… Tm=60oC
2)…-Pro-Flp-Gly-Pro-Flp-Gly-Pro-Flp-Gly-… Tm=78oC
3)…-Gly-Pro-Thr-Gly-Pro-Thr-Gly-Pro-Thr-… Tm=30oC
A)hydrogen bonding
B)inductive effect
C)electrostatic effect
D)electrostatic and Inductive effect are equal
E)hydrogen bonding and inductive effect are equal
1)…-Pro-Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly-… Tm=60oC
2)…-Pro-Flp-Gly-Pro-Flp-Gly-Pro-Flp-Gly-… Tm=78oC
3)…-Gly-Pro-Thr-Gly-Pro-Thr-Gly-Pro-Thr-… Tm=30oC
A)hydrogen bonding
B)inductive effect
C)electrostatic effect
D)electrostatic and Inductive effect are equal
E)hydrogen bonding and inductive effect are equal

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Molecular chaperones bind to unfolded or partially folded polypeptide chains in order to accomplish which of the following?
A)ensure that improper aggregation of hydrophobic segments does not occur
B)engulf the protein in order to ensure that the protein is not damaged by heat denaturation
C)facilitate native folding by exposing hydrophobic segments of the protein as it is synthesized
D)facilitate aggregation of multiple subunits of a protein during synthesis
E)All of the above are accomplished by molecular chaperones.
A)ensure that improper aggregation of hydrophobic segments does not occur
B)engulf the protein in order to ensure that the protein is not damaged by heat denaturation
C)facilitate native folding by exposing hydrophobic segments of the protein as it is synthesized
D)facilitate aggregation of multiple subunits of a protein during synthesis
E)All of the above are accomplished by molecular chaperones.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
What observation about protein refolding or renaturation helped to solidify the connection between primary amino acid sequence and 3-D structure?
A)Spontaneous refolding of proteins into their native state under physiologic conditions.
B)Assisted refolding of proteins into their native state under laboratory conditions.
C)Identification of thermostable proteins than maintain their native state in adverse temperatures.
D)A and B
E)B and C
A)Spontaneous refolding of proteins into their native state under physiologic conditions.
B)Assisted refolding of proteins into their native state under laboratory conditions.
C)Identification of thermostable proteins than maintain their native state in adverse temperatures.
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


