Deck 3: Elements of Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/116
Play
Full screen (f)
Deck 3: Elements of Chemistry
1
A chemical reaction is synonymous to a ________.
A) physical reaction
B) change in phase
C) rise in temperature
D) chemical change
A) physical reaction
B) change in phase
C) rise in temperature
D) chemical change
chemical change
2
In the winter Vermonters make a tasty treat called "sugar on snow" in which they pour boiled-down maple syrup onto a scoop of clean fresh snow. As the syrup hits the snow it forms a delicious taffy. Which of the following changes are involved in the making of sugar on snow?
A) Boiling down the maple syrup involves the evaporation of water.
B) The syrup warms the snow causing it to melt while the syrup becomes more viscous.
C) As the maple syrup is boiled the sugar within the syrup begins to caramelize, which is an example of a chemical change.
D) All of the above changes are involved in the making of sugar on snow.
A) Boiling down the maple syrup involves the evaporation of water.
B) The syrup warms the snow causing it to melt while the syrup becomes more viscous.
C) As the maple syrup is boiled the sugar within the syrup begins to caramelize, which is an example of a chemical change.
D) All of the above changes are involved in the making of sugar on snow.
All of the above changes are involved in the making of sugar on snow.
3
Water and ethanol can be separated by heating the ethanol until it boils away from the water. What type of change is this?
A) a physical change
B) a chemical change
C) a molecular change
D) a decomposition
E) none of the above
A) a physical change
B) a chemical change
C) a molecular change
D) a decomposition
E) none of the above
a physical change
4
A skillet is lined with a thin layer of cooking oil followed by a layer of unpopped popcorn kernels. Upon heating the kernels all pop thereby escaping the skillet. Which of the following physical and/or chemical changes occurred?
A) The water within each kernel is heated to the point that it would turn into water vapor as the kernels popped (physical change).
B) The starches within the kernels are cooked by the high temperatures (chemical change).
C) Both A and B occur.
D) Physical and chemical changes cannot occur without a real chemical reaction taking place.
A) The water within each kernel is heated to the point that it would turn into water vapor as the kernels popped (physical change).
B) The starches within the kernels are cooked by the high temperatures (chemical change).
C) Both A and B occur.
D) Physical and chemical changes cannot occur without a real chemical reaction taking place.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would be considered a chemical property?
A) reactivity towards water
B) melting temperature
C) boiling temperature
D) conductivity
E) flexibility
A) reactivity towards water
B) melting temperature
C) boiling temperature
D) conductivity
E) flexibility

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
6
Classify the following changes as physical or chemical. Wood burns to ashes; water begins to boil; grass grows; a rock is crushed to powder.
A) chemical; physical; chemical; chemical
B) chemical; physical; physical; physical
C) physical; physical; chemical; physical
D) chemical; physical; chemical; physical
A) chemical; physical; chemical; chemical
B) chemical; physical; physical; physical
C) physical; physical; chemical; physical
D) chemical; physical; chemical; physical

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
7
Is the following transformation representative of a physical change or a chemical change? 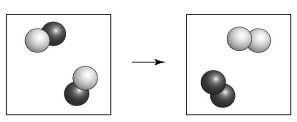
A) chemical change because of the formation of elements
B) physical change because a new material has been formed
C) chemical change because the atoms are connected differently
D) physical change because of a change in phase

A) chemical change because of the formation of elements
B) physical change because a new material has been formed
C) chemical change because the atoms are connected differently
D) physical change because of a change in phase

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following would not be considered a physical property?
A) temperature at which a material catches on fire
B) color
C) conductivity
D) hardness
E) temperature at which a material melts
A) temperature at which a material catches on fire
B) color
C) conductivity
D) hardness
E) temperature at which a material melts

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
9
Based on the information given in the following diagrams, which substance has the lower boiling point: one made from molecule A or one made from molecule B? 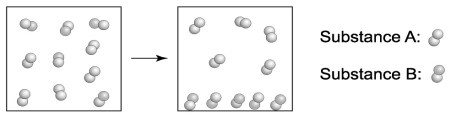
A) molecule A because it is the first to transform into a liquid
B) molecule B because it is first to transform into a liquid
C) molecule A because it remains in the gaseous phase
D) molecule B because it remains in the gaseous phase

A) molecule A because it is the first to transform into a liquid
B) molecule B because it is first to transform into a liquid
C) molecule A because it remains in the gaseous phase
D) molecule B because it remains in the gaseous phase

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is an example of a physical change?
A) water boiling and being converted into steam
B) water being electrolyzed and being converted in hydrogen and oxygen
C) iron metal reacting with oxygen to form rust
D) a candy bar being digested by a student
E) charcoal being converted into ash
A) water boiling and being converted into steam
B) water being electrolyzed and being converted in hydrogen and oxygen
C) iron metal reacting with oxygen to form rust
D) a candy bar being digested by a student
E) charcoal being converted into ash

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
11
The boiling point of methanol is 65°C and the boiling point of ethanol is 78°C. Which of the following statements is true?
A) At 70°C you would have methanol gas and liquid ethanol.
B) At 90°C you would have methanol and ethanol as solids.
C) At 50°C you would have methanol and ethanol as gases.
D) At 40°C the methanol reacts with the ethanol.
E) none of the above
A) At 70°C you would have methanol gas and liquid ethanol.
B) At 90°C you would have methanol and ethanol as solids.
C) At 50°C you would have methanol and ethanol as gases.
D) At 40°C the methanol reacts with the ethanol.
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
12
The element silicon has a melting point of 1,410°C and a boiling point of 2,355°C. It is a weak conductor of electricity, its density is 2.3 grams per cubic centimeter and it easily forms silicon dioxide when exposed to air. Which of the following is a chemical property of silicon?
A) its ability to conduct electricity
B) its density
C) its melting point
D) its ability to react with oxygen to form silicon dioxide
E) C and D
A) its ability to conduct electricity
B) its density
C) its melting point
D) its ability to react with oxygen to form silicon dioxide
E) C and D

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
13
The following image describes what type of change? 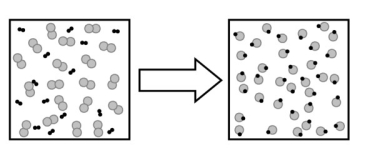
A) a chemical change
B) a physical change
C) a change in state
D) no change
E) an elemental change

A) a chemical change
B) a physical change
C) a change in state
D) no change
E) an elemental change

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not a chemical change?
A) a rock being crushed to powder
B) grass growing
C) grape juice turning into wine
D) a loaf of bread growing mold
E) wood burning to ash
A) a rock being crushed to powder
B) grass growing
C) grape juice turning into wine
D) a loaf of bread growing mold
E) wood burning to ash

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following would not be considered a chemical property?
A) the temperature at which a liquid will boil
B) light sensitivity
C) whether a metal will rust or not
D) whether a material will dissolve in acid or not
E) the tendency of a material to explode
A) the temperature at which a liquid will boil
B) light sensitivity
C) whether a metal will rust or not
D) whether a material will dissolve in acid or not
E) the tendency of a material to explode

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
16
A chemical change involves a change in ________.
A) how atoms are bonded
B) the elements of a molecule
C) phase
D) all of the above
A) how atoms are bonded
B) the elements of a molecule
C) phase
D) all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following would be considered a physical property?
A) density
B) flammability
C) corrosion resistance
D) reactivity towards acid
E) oxygen sensitivity
A) density
B) flammability
C) corrosion resistance
D) reactivity towards acid
E) oxygen sensitivity

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
18
Each night you measure your height just before going to bed. When you arise each morning, you measure your height again and consistently find that you are 1 inch taller than you were the night before but only as tall as you were 24 hours ago! Is what happens to your body in this instance best described as a physical change or a chemical change?
A) chemical change because it involves your body
B) physical change because it readily reverses
C) chemical change because it involves changes in your bone structure
D) physical change because water expands as it freezes
A) chemical change because it involves your body
B) physical change because it readily reverses
C) chemical change because it involves changes in your bone structure
D) physical change because water expands as it freezes

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an example of a chemical change?
A) gasoline being used in the engine of a car producing exhaust
B) water freezing into ice crystals
C) aftershave or perfume on your skin generating a smell
D) a piece of metal expanding when heated, but returning to original size when cooled
E) breaking a glass window
A) gasoline being used in the engine of a car producing exhaust
B) water freezing into ice crystals
C) aftershave or perfume on your skin generating a smell
D) a piece of metal expanding when heated, but returning to original size when cooled
E) breaking a glass window

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
20
The following image describes which type of change? 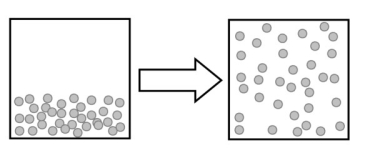
A) a physical change
B) a chemical change
C) an elemental change
D) a change in reactivity
E) no change

A) a physical change
B) a chemical change
C) an elemental change
D) a change in reactivity
E) no change

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these is not a metal?
A) selenium (atomic no. = 34)
B) gallium (atomic no. = 31)
C) lithium (atomic no. = 3)
D) potassium (atomic no. = 19)
E) vanadium (atomic no. = 23)
A) selenium (atomic no. = 34)
B) gallium (atomic no. = 31)
C) lithium (atomic no. = 3)
D) potassium (atomic no. = 19)
E) vanadium (atomic no. = 23)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
22
Oxygen, 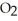 , is certainly good for you. Does it follow that if small amounts of oxygen are good for you then large amounts of oxygen would be especially good for you?
, is certainly good for you. Does it follow that if small amounts of oxygen are good for you then large amounts of oxygen would be especially good for you?
A) Yes. This is the reason patients are given pure (100%) oxygen during medical procedures.
B) Yes. Increased oxygenation of the bloodstream is good for you and can increase your life span.
C) No. Breathing 100% oxygen for extended periods of time can be damaging to the body.
D) No. Large amounts of oxygen will absorb hydrogen from the body and increase the amount of water in the body causing an imbalance in electrolytes.
 , is certainly good for you. Does it follow that if small amounts of oxygen are good for you then large amounts of oxygen would be especially good for you?
, is certainly good for you. Does it follow that if small amounts of oxygen are good for you then large amounts of oxygen would be especially good for you?A) Yes. This is the reason patients are given pure (100%) oxygen during medical procedures.
B) Yes. Increased oxygenation of the bloodstream is good for you and can increase your life span.
C) No. Breathing 100% oxygen for extended periods of time can be damaging to the body.
D) No. Large amounts of oxygen will absorb hydrogen from the body and increase the amount of water in the body causing an imbalance in electrolytes.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
23
Elements that are in the same ________ have a tendency to have very similar chemical properties due to periodic trends.
A) group
B) period
C) textbook
D) compound
E) row
A) group
B) period
C) textbook
D) compound
E) row

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
24
Is aging primarily an example of a physical or chemical change?
A) Aging is an example of a physical change since it involves our physical bodies getting older each day.
B) Aging is an example of a chemical change involving the chemical reformation of our biomolecules.
C) Aging cannot be classified as either a physical or chemical change.
D) None of the above is true.
A) Aging is an example of a physical change since it involves our physical bodies getting older each day.
B) Aging is an example of a chemical change involving the chemical reformation of our biomolecules.
C) Aging cannot be classified as either a physical or chemical change.
D) None of the above is true.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
25
What chemical change occurs when a wax candle burns?
A) The wax near the flame melts.
B) The molten wax is pulled upwards through the wick.
C) The wax within the wick is heated to about 600°C.
D) The heated wax molecules combine with oxygen molecules.
A) The wax near the flame melts.
B) The molten wax is pulled upwards through the wick.
C) The wax within the wick is heated to about 600°C.
D) The heated wax molecules combine with oxygen molecules.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a metal?
A) titanium (atomic no. = 22)
B) sulfur (atomic no. = 16)
C) selenium (atomic no. = 34)
D) xenon (atomic no. = 54)
E) helium (atomic no. = 2)
A) titanium (atomic no. = 22)
B) sulfur (atomic no. = 16)
C) selenium (atomic no. = 34)
D) xenon (atomic no. = 54)
E) helium (atomic no. = 2)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
27
Which atom is largest?
A) Rb
B) K
C) Na
D) Li
E) H
A) Rb
B) K
C) Na
D) Li
E) H

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
28
What are metalloids?
A) elements that have some properties like metals and some like nonmetals
B) elements that are smaller than metals
C) elements found in asteroids
D) elements that are larger than nonmetals
E) elements that have properties different than either the metals or the nonmetals
A) elements that have some properties like metals and some like nonmetals
B) elements that are smaller than metals
C) elements found in asteroids
D) elements that are larger than nonmetals
E) elements that have properties different than either the metals or the nonmetals

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
29
Which of these does not describe a metal at room temperature?
A) gas
B) solid
C) liquid
D) shiny
E) bendable
A) gas
B) solid
C) liquid
D) shiny
E) bendable

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is not the name of a chemical family?
A) heavy metals
B) transition metals
C) alkali metals
D) alkaline-earth metals
E) noble gases
A) heavy metals
B) transition metals
C) alkali metals
D) alkaline-earth metals
E) noble gases

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
31
The atomic symbol for cobalt is ________.
A) Co
B) CO
C) Ct
D) 27
A) Co
B) CO
C) Ct
D) 27

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following describes a nonmetal?
A) poor conductor of electricity
B) shiny
C) malleable
D) ductile
E) good conductor of heat
A) poor conductor of electricity
B) shiny
C) malleable
D) ductile
E) good conductor of heat

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements are in the same period as magnesium (Mg)?
A) Cl
B) Ca
C) Mn
D) Sr
E) none of the above
A) Cl
B) Ca
C) Mn
D) Sr
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these properties describes a metal?
A) conducts heat very well
B) brittle
C) fragile
D) transparent
E) doesn't conduct electricity very well
A) conducts heat very well
B) brittle
C) fragile
D) transparent
E) doesn't conduct electricity very well

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following images could describe an element at the atomic level?
A)
B)
C)
D) none of the images
E) all of the images
A)

B)

C)

D) none of the images
E) all of the images

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
36
Which atom is smallest?
A) Be
B) Mg
C) Ca
D) Sr
E) All are the same size.
A) Be
B) Mg
C) Ca
D) Sr
E) All are the same size.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements best describes an element?
A) a material consisting of only one type of atom
B) a material that is pure
C) a material that has consistent physical properties
D) a material with more than one type of atom
E) a material that has consistent chemical properties
A) a material consisting of only one type of atom
B) a material that is pure
C) a material that has consistent physical properties
D) a material with more than one type of atom
E) a material that has consistent chemical properties

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of iron weighs more after it rusts because ________.
A) it has expanded into a greater volume
B) rust contains twice as many iron atoms
C) of the additional oxygen it contains
D) Wrong. Iron actually weighs less after it rusts
A) it has expanded into a greater volume
B) rust contains twice as many iron atoms
C) of the additional oxygen it contains
D) Wrong. Iron actually weighs less after it rusts

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following elements are in the same group as silicon (Si)?
A) C
B) P
C) As
D) B
E) none of the above
A) C
B) P
C) As
D) B
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a metalloid?
A) antimony (atomic no. = 51)
B) zinc (atomic no. = 30)
C) iodine (atomic no. = 53)
D) uranium (atomic no. = 92)
E) sulfur (atomic no. = 16)
A) antimony (atomic no. = 51)
B) zinc (atomic no. = 30)
C) iodine (atomic no. = 53)
D) uranium (atomic no. = 92)
E) sulfur (atomic no. = 16)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement best describes a compound?
A) a material that is made up of a combination of atoms bonded together
B) a mixture of more than one element
C) a mixture of atoms
D) a material that is made up of a single type of atom
E) none of the above
A) a material that is made up of a combination of atoms bonded together
B) a mixture of more than one element
C) a mixture of atoms
D) a material that is made up of a single type of atom
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
42
Strontium, Sr (number 38), is especially dangerous to humans because it tends to accumulate in calcium-dependent bone marrow tissues (calcium, Ca, number 20). This fact relates to the organization of the periodic table in that strontium and calcium are both ________.
A) metals
B) in group 2 of the periodic table
C) made of relatively large atoms
D) soluble in water
A) metals
B) in group 2 of the periodic table
C) made of relatively large atoms
D) soluble in water

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
43
Helium, He, is a nonmetallic gas and the second element in the periodic table. Rather than being placed adjacent to hydrogen, H, however, helium is placed on the far right of the table because ________.
A) hydrogen and helium repel one another
B) the sizes of their atoms are vastly different
C) they come from different sources
D) helium is most similar to other group 18 elements
A) hydrogen and helium repel one another
B) the sizes of their atoms are vastly different
C) they come from different sources
D) helium is most similar to other group 18 elements

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
44
Which chemical family is composed almost entirely of synthetic elements?
A) the actinides
B) the lanthanides
C) the halogens
D) all of the above
E) none of the above
A) the actinides
B) the lanthanides
C) the halogens
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
45
Germanium, Ge (number 32), computer chips operate faster than silicon, Si (number 14), computer chips. So how might a gallium, Ga (number 31), chip compare with a germanium chip?
A) A gallium chip would be even faster because the gallium is more metallic.
B) A gallium chip would be slower because its electrons are more loosely held.
C) Gallium is located just below aluminum, which is widely known to be an electrical insulator.
D) Gallium is more nonmetallic and so it does not conduct electrons very well.
A) A gallium chip would be even faster because the gallium is more metallic.
B) A gallium chip would be slower because its electrons are more loosely held.
C) Gallium is located just below aluminum, which is widely known to be an electrical insulator.
D) Gallium is more nonmetallic and so it does not conduct electrons very well.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following elements is a gas at room temperature?
A) argon (Ar)
B) lead (Pb)
C) cesium (Cs)
D) indium (In)
E) lithium (Li)
A) argon (Ar)
B) lead (Pb)
C) cesium (Cs)
D) indium (In)
E) lithium (Li)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
47
What happens to the properties of elements across any period of the periodic table?
A) The elements tend to become more metallic in nature since they are increasing in atomic number.
B) The elements get much larger in size because of the addition of more protons and electrons.
C) The properties of the elements gradually change across any period of the periodic table.
D) All of the above are true.
A) The elements tend to become more metallic in nature since they are increasing in atomic number.
B) The elements get much larger in size because of the addition of more protons and electrons.
C) The properties of the elements gradually change across any period of the periodic table.
D) All of the above are true.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following physical properties would you expect for krypton (Kr)?
A) a gas at room temperature
B) hard
C) brittle
D) shiny
E) conducts electricity
A) a gas at room temperature
B) hard
C) brittle
D) shiny
E) conducts electricity

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
49
The repeating trends that take place when examining the elements are called ________.
A) periodicity
B) the family cycle
C) the metal shift
D) a group conscience
E) none of the above
A) periodicity
B) the family cycle
C) the metal shift
D) a group conscience
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
50
A compound is represented by the ________.
A) elemental formula
B) periodic table
C) chemical formula
D) compoundic symbol
A) elemental formula
B) periodic table
C) chemical formula
D) compoundic symbol

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
51
Why isn't dirt listed in the periodic table?
A) The periodic table lists only elements made of one kind of material. Dirt is a mixture of elements and compounds.
B) Elements like dirt and air are so common that there is no need to list them in the periodic table.
C) Dirt IS listed in the periodic table but is not easily recognized because it is listed as one of the rare earths with its old scientific name, dysprosium, symbol Dy.
D) None of the above is true.
A) The periodic table lists only elements made of one kind of material. Dirt is a mixture of elements and compounds.
B) Elements like dirt and air are so common that there is no need to list them in the periodic table.
C) Dirt IS listed in the periodic table but is not easily recognized because it is listed as one of the rare earths with its old scientific name, dysprosium, symbol Dy.
D) None of the above is true.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following elements is an alkali metal?
A) argon (Ar)
B) lead (Pb)
C) cerium (Cr)
D) indium (In)
E) lithium (Li)
A) argon (Ar)
B) lead (Pb)
C) cerium (Cr)
D) indium (In)
E) lithium (Li)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
53
About how many elements do you have access to as a consumer of market goods?
A) none
B) one
C) ten
D) one hundred
A) none
B) one
C) ten
D) one hundred

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
54
Should the periodic table be memorized? Why or why not?
A) Yes. Like the alphabet, we need to memorize the periodic table in order to easily write the language of chemistry.
B) Yes. Without memorizing the periodic table, one would not have any real understanding of how and why chemical compounds are put together.
C) No. The periodic table changes every year. Memorizing it would be a waste of time.
D) No. The periodic table is a reference to be used, not memorized.
A) Yes. Like the alphabet, we need to memorize the periodic table in order to easily write the language of chemistry.
B) Yes. Without memorizing the periodic table, one would not have any real understanding of how and why chemical compounds are put together.
C) No. The periodic table changes every year. Memorizing it would be a waste of time.
D) No. The periodic table is a reference to be used, not memorized.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following elements is a halogen?
A) argon (Ar)
B) lead (Pb)
C) chlorine (Cl)
D) indium (In)
E) lithium (Li)
A) argon (Ar)
B) lead (Pb)
C) chlorine (Cl)
D) indium (In)
E) lithium (Li)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
56
Which element would have chemical properties the most similar to chlorine (Cl)?
A) Br
B) O
C) Ar
D) S
E) Na
A) Br
B) O
C) Ar
D) S
E) Na

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following elements will most likely be shiny and flexible?
A) rhodium (Rh)
B) hydrogen (H)
C) selenium (Se)
D) iodine (I)
E) silicon (Si)
A) rhodium (Rh)
B) hydrogen (H)
C) selenium (Se)
D) iodine (I)
E) silicon (Si)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following elements is a transition metal?
A) xenon (Xe)
B) lead (Pb)
C) chlorine (Cl)
D) silver (Ag)
E) lithium (Li)
A) xenon (Xe)
B) lead (Pb)
C) chlorine (Cl)
D) silver (Ag)
E) lithium (Li)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
59
The oldest known elements in the periodic table are the ones ________.
A) with the lowest atomic numbers
B) with the highest atomic numbers
C) that match their Latin names
D) with atomic symbols that match their modern names
A) with the lowest atomic numbers
B) with the highest atomic numbers
C) that match their Latin names
D) with atomic symbols that match their modern names

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following elements is in the fourth period?
A) chromium (Cr)
B) hydrogen (H)
C) beryllium(Be)
D) carbon (C)
E) zirconium (Zr)
A) chromium (Cr)
B) hydrogen (H)
C) beryllium(Be)
D) carbon (C)
E) zirconium (Zr)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
61
Each circle represents an atom. Which of the following boxes contains an element? A compound? A mixture? 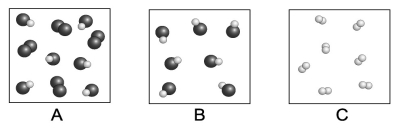
A) element: A, C; compound: A, B, C; mixture: A, B
B) element: C; compound: A, B; mixture: B
C) element: A, C; compound: A, B; mixture: A
D) element: A, C; compound: A, B; mixture: A, B

A) element: A, C; compound: A, B, C; mixture: A, B
B) element: C; compound: A, B; mixture: B
C) element: A, C; compound: A, B; mixture: A
D) element: A, C; compound: A, B; mixture: A, B

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
62
The systematic names for water, ammonia, and methane are dihydrogen monoxide, 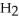 O; trihydrogen nitride, N
O; trihydrogen nitride, N 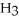 ; and tetrahydrogen carbide, C
; and tetrahydrogen carbide, C 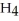 . Why do most people, including chemists, prefer to use the common names for these compounds?
. Why do most people, including chemists, prefer to use the common names for these compounds?
A) The common names are shorter and easier to pronounce.
B) These compounds are encountered frequently.
C) The common names are more widely known.
D) all of the above
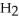 O; trihydrogen nitride, N
O; trihydrogen nitride, N 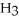 ; and tetrahydrogen carbide, C
; and tetrahydrogen carbide, C 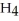 . Why do most people, including chemists, prefer to use the common names for these compounds?
. Why do most people, including chemists, prefer to use the common names for these compounds?A) The common names are shorter and easier to pronounce.
B) These compounds are encountered frequently.
C) The common names are more widely known.
D) all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following boxes represents a compound? 

 A B C
A B C
A) only A
B) only B
C) only C
D) both A and C


 A B C
A B CA) only A
B) only B
C) only C
D) both A and C

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
64
What is the name of the following compound? SF3
A) sulfur trifluoride
B) sulfur fluoride
C) trifluorosulphide
D) fluorine sulphide
E) none of the above
A) sulfur trifluoride
B) sulfur fluoride
C) trifluorosulphide
D) fluorine sulphide
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
65
How many different elements are in the compound C6H12O6?
A) 3
B) 6
C) 24
D) All of the elements are the same.
A) 3
B) 6
C) 24
D) All of the elements are the same.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
66
How many atoms of Oxygen (O) are in HClO4?
A) 5
B) 2
C) 3
D) 4
E) 1
A) 5
B) 2
C) 3
D) 4
E) 1

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
67
What is the name of the following compound? CaCl2
A) calcium chloride
B) carbon chloride
C) dichlorocalcium
D) calc two
E) dicalcium chloride
A) calcium chloride
B) carbon chloride
C) dichlorocalcium
D) calc two
E) dicalcium chloride

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
68
If you have one molecule of CO2, how many molecules of O2 does it contain?
A) None, O2 is a different molecule than CO2.
B) One, CO2 is a mixture of C and O2.
C) Two, CO2 is a mixture of C and 2 O.
D) Three, CO2 contains three molecules.
E) none of the above
A) None, O2 is a different molecule than CO2.
B) One, CO2 is a mixture of C and O2.
C) Two, CO2 is a mixture of C and 2 O.
D) Three, CO2 contains three molecules.
E) none of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
69
Oxygen atoms are used to make water molecules. Does this mean that oxygen, 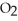 , and water,
, and water, 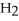 O, have similar properties?
O, have similar properties?
A) Yes, and this explains how fish are able to breathe water.
B) Yes, but that their properties are similar is only a coincidence.
C) No, but their similar properties are only a coincidence.
D) No, compounds are uniquely different from the elements from which they're made.
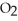 , and water,
, and water, 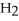 O, have similar properties?
O, have similar properties?A) Yes, and this explains how fish are able to breathe water.
B) Yes, but that their properties are similar is only a coincidence.
C) No, but their similar properties are only a coincidence.
D) No, compounds are uniquely different from the elements from which they're made.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
70
The following image represents which kind of matter? 
A) a mixture
B) a compound
C) an element
D) none of the above
E) all of the above

A) a mixture
B) a compound
C) an element
D) none of the above
E) all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
71
The four substances below are either elements or compounds. Which are which?
A) elements: ; compounds:
; compounds: 
B) elements:
 ; compounds:
; compounds: 
C) elements: ; compounds:
; compounds: 
D) elements: ; compounds:
; compounds: 
A) elements:
 ; compounds:
; compounds: 
B) elements:

 ; compounds:
; compounds: 
C) elements:
 ; compounds:
; compounds: 
D) elements:
 ; compounds:
; compounds: 

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
72
What is the difference between a compound and a mixture?
A) A mixture can be physically separated into its components; a compound cannot be physically separated into its components.
B) A compound can be physically separated into its components; a mixture cannot be physically separated into its components.
C) A compound is just a mixture of elements.
D) The components of a mixture do not have the same properties individually as they do when mixed.
E) The components of a compound have the same properties individually as they do when mixed.
A) A mixture can be physically separated into its components; a compound cannot be physically separated into its components.
B) A compound can be physically separated into its components; a mixture cannot be physically separated into its components.
C) A compound is just a mixture of elements.
D) The components of a mixture do not have the same properties individually as they do when mixed.
E) The components of a compound have the same properties individually as they do when mixed.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
73
The following image represents which kind of matter? 
A) a compound
B) a mixture
C) an element
D) none of the above
E) all of the above

A) a compound
B) a mixture
C) an element
D) none of the above
E) all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
74
How many atoms are in one molecule of Na2SO4?
A) 7
B) 2
C) 4
D) 3
E) 24
A) 7
B) 2
C) 4
D) 3
E) 24

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
75
A combination of two or more substances in which they no longer retain their chemical properties is called a(n) ________.
A) mixture
B) compound
C) heterogeneous mixture
D) periodic trend
E) suspension
A) mixture
B) compound
C) heterogeneous mixture
D) periodic trend
E) suspension

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
76
If you have two molecules of TiO2, how many oxygen atoms would you have?
A) 4
B) 2
C) 3
D) 6
E) none
A) 4
B) 2
C) 3
D) 6
E) none

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
77
What is the name of the following compound? NaF
A) sodium fluoride
B) natural fosfate
C) natrium fluoride
D) nitrogen afleck
E) sodium phosphide
A) sodium fluoride
B) natural fosfate
C) natrium fluoride
D) nitrogen afleck
E) sodium phosphide

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is a mixture?
A) air
B) gold
C) salt
D) iron
E) helium
A) air
B) gold
C) salt
D) iron
E) helium

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
79
If you eat metallic sodium or inhale chlorine gas, you stand a strong chance of dying. Let these two elements react with each other, however, and you can safely sprinkle the compound on your popcorn for better taste. What is going on?
A) After these two elements react they lose the potential energy to cause harm.
B) All elements are inherently dangerous
C) Sodium and chlorine from the elemental form is more concentrated than the sodium and chlorine we get from sodium chloride.
D) Sodium chloride has nothing in common with the properties of the elements sodium and chlorine.
A) After these two elements react they lose the potential energy to cause harm.
B) All elements are inherently dangerous
C) Sodium and chlorine from the elemental form is more concentrated than the sodium and chlorine we get from sodium chloride.
D) Sodium chloride has nothing in common with the properties of the elements sodium and chlorine.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
80
What is the name of the following compound? CO2
A) carbon dioxide
B) dicobalt
C) dioxocarbon
D) calcium oxide
E) calcium dioxide
A) carbon dioxide
B) dicobalt
C) dioxocarbon
D) calcium oxide
E) calcium dioxide

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck


