Deck 11: Oxidations and Reductions Charge the World
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/138
Play
Full screen (f)
Deck 11: Oxidations and Reductions Charge the World
1
What is a reduction?
A) the gain of electrons
B) the reduction of the number of electrons
C) the loss of an electron from the valence shell
D) the reaction of oxygen with a reductant
E) the formation of red compounds in the presence of an oxidant
A) the gain of electrons
B) the reduction of the number of electrons
C) the loss of an electron from the valence shell
D) the reaction of oxygen with a reductant
E) the formation of red compounds in the presence of an oxidant
the gain of electrons
2
Which of the following species is undergoing reduction? 2 CuBr → 2Cu + Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2
Cu+
3
Which of the following statements about oxidation and reduction reactions is true?
A) More than one electron can be transferred in an oxidation and reduction reaction.
B) In a half-reaction, only half of an electron is transferred.
C) Only neutral atoms are formed in oxidation and reduction reactions.
D) A half-reaction is only used for reduction reactions.
E) None of the above are true.
A) More than one electron can be transferred in an oxidation and reduction reaction.
B) In a half-reaction, only half of an electron is transferred.
C) Only neutral atoms are formed in oxidation and reduction reactions.
D) A half-reaction is only used for reduction reactions.
E) None of the above are true.
More than one electron can be transferred in an oxidation and reduction reaction.
4
How many electrons are gained or lost in the following half-reaction? 2 Na → 2 Na+
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following species is the reducing agent? 2 CuBr → 2Cu + Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
6
How many electrons are gained or lost in the following half-reaction? Na → Na+
A) 1 electron is lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 1 electron is gained
E) 2 electrons are gained
A) 1 electron is lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 1 electron is gained
E) 2 electrons are gained

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
7
How many electrons are gained or lost in the following half-reaction? Zn → Zn2+
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following materials is most likely to undergo reduction?
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is untrue about oxidation and reduction processes?
A) An oxidation can happen without a reduction.
B) It involves the exchange of electrons.
C) Often ions are generated or consumed.
D) Electrons are often involved in oxidation and reduction reactions.
E) All of the above are true.
A) An oxidation can happen without a reduction.
B) It involves the exchange of electrons.
C) Often ions are generated or consumed.
D) Electrons are often involved in oxidation and reduction reactions.
E) All of the above are true.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
10
How many electrons are gained or lost in the following half-reaction? C → C4-
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following species is the oxidizing agent? 2 CuBr → 2Cu + Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
12
The elements that make the best reducing agents are the ________.
A) group 18 noble gases
B) group 17 halogens
C) transition metals
D) group 1 alkali metals
A) group 18 noble gases
B) group 17 halogens
C) transition metals
D) group 1 alkali metals

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following materials is most likely to act as an oxidizing agent?
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
14
How many electrons are gained or lost in the following half-reaction? Cl2 → 2 Cl-
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained
A) 2 electrons are lost
B) 1/2 electron is gained
C) 1/2 electron is lost
D) 2 electrons are gained
E) 4 electrons are gained

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following materials is most likely to act as a reducing agent?
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the half-reactions below would be complementary, balance the following half-reaction, and be chemically reasonable? Br2 → 2 Br-
A) 2 K → 2 K+
B) K+ → K
C) K → K+
D) 2 K+ → 2 K
E) K → K2+
A) 2 K → 2 K+
B) K+ → K
C) K → K+
D) 2 K+ → 2 K
E) K → K2+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following materials is most likely to undergo oxidation?
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B
A) Na
B) Na+
C) Cl2
D) Cl-
E) both A and B

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following species is undergoing oxidation? 2 CuBr → 2Cu + Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2
A) Cu
B) CuBr
C) Cu+
D) Br-
E) Br2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
19
What is oxidation?
A) the loss of electrons
B) the reaction with oxygen
C) the reduction of oxygen
D) the gaining of electrons
E) the addition of an electron to the valence shell
A) the loss of electrons
B) the reaction with oxygen
C) the reduction of oxygen
D) the gaining of electrons
E) the addition of an electron to the valence shell

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the half-reactions below would be complementary and balance the following half-reaction? Zn+2 → Zn
A) Cu → Cu2+
B) K+ → K
C) 2 Cu → 2 Cu2+
D) 2 K+ → 2 K
E) Cl2 → 2 Cl-
A) Cu → Cu2+
B) K+ → K
C) 2 Cu → 2 Cu2+
D) 2 K+ → 2 K
E) Cl2 → 2 Cl-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
21
In the above diagram, which atom behaves as the oxidizing agent, the lighter colored atom or the darker colored atom?
A) Both atoms behave as the oxidizing agent.
B) Neither of the atoms behave as the oxidizing agent.
C) The darker colored atom behaves as the oxidizing agent.
D) The lighter colored atom behaves as the oxidizing agent.
A) Both atoms behave as the oxidizing agent.
B) Neither of the atoms behave as the oxidizing agent.
C) The darker colored atom behaves as the oxidizing agent.
D) The lighter colored atom behaves as the oxidizing agent.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
22
Based on their relative positions in the periodic table, which might you expect to be a stronger oxidizing agent, chlorine or fluorine? Why?
A) Chlorine should behave as a stronger oxidizing agent because it has a smaller effective nuclear charge in its outermost shell.
B) Fluorine should behave as a stronger oxidizing agent because it has a smaller effective nuclear charge in its outermost shell.
C) Chlorine should behave as a stronger oxidizing agent because it has a greater effective nuclear charge in its outermost shell.
D) Fluorine should behave as a stronger oxidizing agent because it has a greater effective nuclear charge in its outermost shell.
A) Chlorine should behave as a stronger oxidizing agent because it has a smaller effective nuclear charge in its outermost shell.
B) Fluorine should behave as a stronger oxidizing agent because it has a smaller effective nuclear charge in its outermost shell.
C) Chlorine should behave as a stronger oxidizing agent because it has a greater effective nuclear charge in its outermost shell.
D) Fluorine should behave as a stronger oxidizing agent because it has a greater effective nuclear charge in its outermost shell.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
23
When lightning strikes, nitrogen molecules, 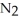 , and oxygen molecules,
, and oxygen molecules, 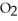 , in the air react to form nitrates,
, in the air react to form nitrates, 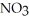 ⁻, which come down in the rain to help fertilize the soil. Is this this an example of oxidation or reduction?
⁻, which come down in the rain to help fertilize the soil. Is this this an example of oxidation or reduction?
A) The formation of nitrates is an example of oxidation.
B) The formation of nitrates is an example of reduction.
C) Both. The nitrogen is oxidized as it reacts with the oxygen while the oxygen is reduced.
D) Neither. Although the bonds of both the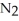 and
and 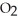 molecules are broken to form the
molecules are broken to form the 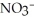 , neither oxidation nor reduction occurs.
, neither oxidation nor reduction occurs.
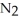 , and oxygen molecules,
, and oxygen molecules, 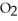 , in the air react to form nitrates,
, in the air react to form nitrates, 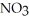 ⁻, which come down in the rain to help fertilize the soil. Is this this an example of oxidation or reduction?
⁻, which come down in the rain to help fertilize the soil. Is this this an example of oxidation or reduction?A) The formation of nitrates is an example of oxidation.
B) The formation of nitrates is an example of reduction.
C) Both. The nitrogen is oxidized as it reacts with the oxygen while the oxygen is reduced.
D) Neither. Although the bonds of both the
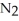 and
and 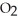 molecules are broken to form the
molecules are broken to form the 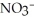 , neither oxidation nor reduction occurs.
, neither oxidation nor reduction occurs.
Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
24
What correlation might you expect between an element's electronegativity and its ability to behave as an oxidizing agent? How about its ability to behave as a reducing agent?
A) Atoms with great electronegativity tend to behave as strong oxidizing agents and weak reducing agents.
B) Atoms with great electronegativity tend to behave as weak oxidizing agents and weak reducing agents.
C) Atoms with great electronegativity tend to behave as strong oxidizing agents and strong reducing agents.
D) Atoms with great electronegativity tend to behave as weak oxidizing agents and strong reducing agents.
A) Atoms with great electronegativity tend to behave as strong oxidizing agents and weak reducing agents.
B) Atoms with great electronegativity tend to behave as weak oxidizing agents and weak reducing agents.
C) Atoms with great electronegativity tend to behave as strong oxidizing agents and strong reducing agents.
D) Atoms with great electronegativity tend to behave as weak oxidizing agents and strong reducing agents.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
25
What might the relationship be between an element's ionization energy and its ability to behave as an oxidizing agent?
A) As the ionization energy increases, the ability of an element to act as an oxidizing agent increases.
B) As the ionization energy increases, the ability of an element to act as an oxidizing agent decreases.
C) As the ionization energy increases, the ability of an element to act as an oxidizing agent stays the same.
D) none of the above
A) As the ionization energy increases, the ability of an element to act as an oxidizing agent increases.
B) As the ionization energy increases, the ability of an element to act as an oxidizing agent decreases.
C) As the ionization energy increases, the ability of an element to act as an oxidizing agent stays the same.
D) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
26
The general chemical equation for photosynthesis is shown below. Through this reaction, are the oxygens in the water molecules, H2O, oxidized or reduced? 6 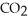 + 6
+ 6 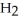 O →
O → 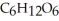 + 6
+ 6 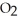
A) The oxygens of the water molecules are oxidized.
B) The oxygens of the water molecules are reduced.
C) The oxygens of some of these water molecules are oxidized while others are reduced.
D) The oxygens of the water molecules are neither oxidized nor reduced.
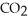 + 6
+ 6 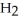 O →
O → 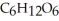 + 6
+ 6 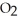
A) The oxygens of the water molecules are oxidized.
B) The oxygens of the water molecules are reduced.
C) The oxygens of some of these water molecules are oxidized while others are reduced.
D) The oxygens of the water molecules are neither oxidized nor reduced.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
27
In the above diagram, which atom is oxidized, the darker colored atom or the lighter colored atom?
A) Both of the atoms are oxidized.
B) Neither of the atoms is oxidized.
C) Only the darker colored atom is oxidized.
D) Only the lighter colored atom is oxidized.
A) Both of the atoms are oxidized.
B) Neither of the atoms is oxidized.
C) Only the darker colored atom is oxidized.
D) Only the lighter colored atom is oxidized.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following molecules. What is the relationship between the degree to which the molecule is oxidized and its polarity? 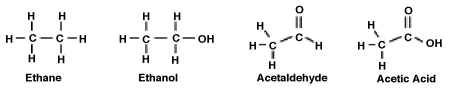
A) The greater the degree of oxidation, the more polar the molecule. Therefore, acetic acid is both the most oxidized and the most polar.
B) The greater the degree of oxidation, the less polar the molecule. Therefore, ethane is both the most oxidized and the least polar.
C) Only acetaldehyde and acetic acid are oxidized and polar because they contain a double-bonded carbon-oxygen bond, C=O. Of the two, acetaldehyde is more polar.
D) There is no correlation between degree to which a molecule is oxidized and its polarity.

A) The greater the degree of oxidation, the more polar the molecule. Therefore, acetic acid is both the most oxidized and the most polar.
B) The greater the degree of oxidation, the less polar the molecule. Therefore, ethane is both the most oxidized and the least polar.
C) Only acetaldehyde and acetic acid are oxidized and polar because they contain a double-bonded carbon-oxygen bond, C=O. Of the two, acetaldehyde is more polar.
D) There is no correlation between degree to which a molecule is oxidized and its polarity.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
29
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ 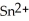 + ____ Ag → ____ Sn + ____ Ag⁺
+ ____ Ag → ____ Sn + ____ Ag⁺
A) 1, 2, 1, 2
B) 1, 1, 1, 2
C) 1, 2, 2, 2
D) 1, 1, 2, 1
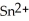 + ____ Ag → ____ Sn + ____ Ag⁺
+ ____ Ag → ____ Sn + ____ Ag⁺A) 1, 2, 1, 2
B) 1, 1, 1, 2
C) 1, 2, 2, 2
D) 1, 1, 2, 1

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
30
Hydrogen sulfide, H2S, burns in the presence of oxygen, 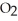 , to produce water,
, to produce water, 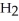 O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2
O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2 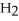 S + 3
S + 3 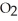 → 2
→ 2 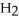 O + 2
O + 2 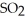
A) Sulfur is reduced.
B) Sulfur is oxidized
C) Sulfur is both oxidized and reduced.
D) Sulfur is neither oxidized nor reduced.
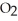 , to produce water,
, to produce water, 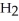 O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2
O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2 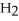 S + 3
S + 3 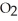 → 2
→ 2 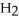 O + 2
O + 2 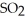
A) Sulfur is reduced.
B) Sulfur is oxidized
C) Sulfur is both oxidized and reduced.
D) Sulfur is neither oxidized nor reduced.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
31
What might the relationship be between an element's electronegativity and its ability to behave as an oxidizing agent?
A) As the electronegativity increases the ability of an element to act as an oxidizing agent increases.
B) As the electronegativity increases the ability of an element to act as an oxidizing agent decreases.
C) As the electronegativity increases the ability of an element to act as an oxidizing agent stays the same.
D) none of the above
A) As the electronegativity increases the ability of an element to act as an oxidizing agent increases.
B) As the electronegativity increases the ability of an element to act as an oxidizing agent decreases.
C) As the electronegativity increases the ability of an element to act as an oxidizing agent stays the same.
D) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
32
Which element is closer to the upper right corner of the periodic table, the one indicated by the lighter colored atoms or the darker colored atoms? 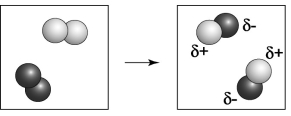
A) the element indicated by the darker color
B) the element indicated by the lighter color
C) More information is needed in order to determine which element is closer to the upper right of the periodic table.
D) Both the lighter and darker colored elements are likely found at the upper right corner of the periodic table.

A) the element indicated by the darker color
B) the element indicated by the lighter color
C) More information is needed in order to determine which element is closer to the upper right of the periodic table.
D) Both the lighter and darker colored elements are likely found at the upper right corner of the periodic table.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
33
What correlation might you expect between an element's ionization energy and its ability to behave as an oxidizing agent? How about its ability to behave as a reducing agent?
A) An element with a high ionization energy tends to behave as a weak oxidizing agent and strong reducing agent.
B) An element with a high ionization energy tends to behave as a strong oxidizing agent and strong reducing agent.
C) An element with a high ionization energy tends to behave as a weak oxidizing agent and weak reducing agent.
D) An element with a high ionization energy tends to behave as a strong oxidizing agent and weak reducing agent.
A) An element with a high ionization energy tends to behave as a weak oxidizing agent and strong reducing agent.
B) An element with a high ionization energy tends to behave as a strong oxidizing agent and strong reducing agent.
C) An element with a high ionization energy tends to behave as a weak oxidizing agent and weak reducing agent.
D) An element with a high ionization energy tends to behave as a strong oxidizing agent and weak reducing agent.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
34
What element is oxidized in the following equation and what element is reduced? 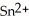 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺
A) The tin ion,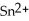 , is oxidized, while the silver, Ag, is reduced.
, is oxidized, while the silver, Ag, is reduced.
B) The tin ion,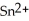 , is reduced, while the silver, Ag, is oxidized.
, is reduced, while the silver, Ag, is oxidized.
C) Both the tin ion,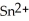 , and the silver, Ag, are reduced.
, and the silver, Ag, are reduced.
D) Both the tin ion,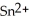 , and the silver, Ag, are oxidized.
, and the silver, Ag, are oxidized.
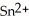 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺A) The tin ion,
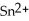 , is oxidized, while the silver, Ag, is reduced.
, is oxidized, while the silver, Ag, is reduced.B) The tin ion,
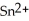 , is reduced, while the silver, Ag, is oxidized.
, is reduced, while the silver, Ag, is oxidized.C) Both the tin ion,
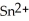 , and the silver, Ag, are reduced.
, and the silver, Ag, are reduced.D) Both the tin ion,
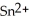 , and the silver, Ag, are oxidized.
, and the silver, Ag, are oxidized.
Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
35
The general chemical equation for photosynthesis is shown below. Through this reaction is the carbon oxidized or reduced? How can you tell? 6 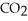 + 6
+ 6 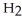 O →
O → 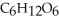 + 6
+ 6 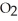
A) Oxidized, since carbon is in the +4 oxidation state in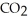 but in the +6 oxidation state in the product,
but in the +6 oxidation state in the product, 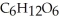 , glucose.
, glucose.
B) Reduced, since in carbon dioxide there are two oxygen atoms for every one carbon but within the product,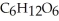 , (glucose), there is only one oxygen for every one carbon.
, (glucose), there is only one oxygen for every one carbon.
C) Neither, since carbon does not change its oxidation state and is neither oxidized nor reduced.
D) Both, since the carbon atoms within the glucose molecules display two different charge states.
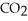 + 6
+ 6 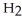 O →
O → 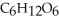 + 6
+ 6 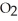
A) Oxidized, since carbon is in the +4 oxidation state in
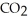 but in the +6 oxidation state in the product,
but in the +6 oxidation state in the product, 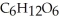 , glucose.
, glucose.B) Reduced, since in carbon dioxide there are two oxygen atoms for every one carbon but within the product,
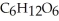 , (glucose), there is only one oxygen for every one carbon.
, (glucose), there is only one oxygen for every one carbon.C) Neither, since carbon does not change its oxidation state and is neither oxidized nor reduced.
D) Both, since the carbon atoms within the glucose molecules display two different charge states.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
36
How does an atom's electronegativity relate to its ability to become oxidized?
A) The greater the electronegativity of an atom, the greater its ability to become oxidized.
B) The lower the electronegativity of an atom, the lower its ability to become oxidized.
C) The greater the electronegativity of an atom, the lower its ability to become oxidized.
D) Electronegativity does not effect the atom's ability to become oxidized.
A) The greater the electronegativity of an atom, the greater its ability to become oxidized.
B) The lower the electronegativity of an atom, the lower its ability to become oxidized.
C) The greater the electronegativity of an atom, the lower its ability to become oxidized.
D) Electronegativity does not effect the atom's ability to become oxidized.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
37
What element is behaves as the oxidizing agent in the following equation and what element behaves as the reducing agent? 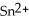 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺
A) The tin ion,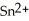 , is the oxidizing agent while silver, Ag, is the reducing agent.
, is the oxidizing agent while silver, Ag, is the reducing agent.
B) The tin ion, Sn2+, is the reducing agent while silver, Ag, is the oxidizing agent.
C) The tin, Sn, is the reducing agent while silver ion, Ag⁺, is the oxidizing agent.
D) The tin, Sn, is the oxidizing agent while silver ion,Ag⁺, is the reducing agent.
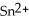 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺A) The tin ion,
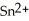 , is the oxidizing agent while silver, Ag, is the reducing agent.
, is the oxidizing agent while silver, Ag, is the reducing agent.B) The tin ion, Sn2+, is the reducing agent while silver, Ag, is the oxidizing agent.
C) The tin, Sn, is the reducing agent while silver ion, Ag⁺, is the oxidizing agent.
D) The tin, Sn, is the oxidizing agent while silver ion,Ag⁺, is the reducing agent.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
38
Iron atoms, Fe, are better reducing agents than copper ions, 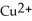 . In which direction do electrons flow when an iron nail is submerged in a solution of
. In which direction do electrons flow when an iron nail is submerged in a solution of 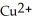 ions?
ions?
A) Electrons cannot flow from the nail to the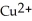 ions because there is no conductor between the two.
ions because there is no conductor between the two.
B) The electrons flow from the submerged nail to the copper ions in solution.
C) The electrons flow from the positive charge on the nail to the negative charge on the nail.
D) The electrons flow from the copper ions in solution to the submerged nail.
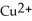 . In which direction do electrons flow when an iron nail is submerged in a solution of
. In which direction do electrons flow when an iron nail is submerged in a solution of 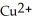 ions?
ions?A) Electrons cannot flow from the nail to the
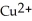 ions because there is no conductor between the two.
ions because there is no conductor between the two.B) The electrons flow from the submerged nail to the copper ions in solution.
C) The electrons flow from the positive charge on the nail to the negative charge on the nail.
D) The electrons flow from the copper ions in solution to the submerged nail.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
39
Clorox is a laundry bleaching agent used to remove stains from white clothes. Suggest why the name begins with Clor- and ends with -ox.
A) Clor- because it has chlorine, -ox because it is as strong as an ox.
B) The active ingredient contains chlorine atoms, which behave as strong oxidizing agents.
C) Chlor- because it has chlorine ions, -ox because it is reduced.
D) Chlor- because it has chlorine ions, -ox because it uses oxygen.
A) Clor- because it has chlorine, -ox because it is as strong as an ox.
B) The active ingredient contains chlorine atoms, which behave as strong oxidizing agents.
C) Chlor- because it has chlorine ions, -ox because it is reduced.
D) Chlor- because it has chlorine ions, -ox because it uses oxygen.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
40
For each of the following unbalanced equations, which is depicted: oxidation or reduction? a) Cr → 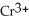 b) Sn →
b) Sn → 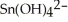
A) a) oxidation; b) reduction
B) a) reduction; b) oxidation
C) a) reduction; b) reduction
D) a) oxidation; b) oxidation
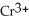 b) Sn →
b) Sn → 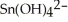
A) a) oxidation; b) reduction
B) a) reduction; b) oxidation
C) a) reduction; b) reduction
D) a) oxidation; b) oxidation

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
41
What is the purpose of the salt bridge in a voltaic cell?
A) to prevent any further migration of electrons through the wire
B) to allow for the build up of positively charged ions in one container and negatively charged ions in the other container
C) to allow the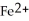 and the
and the 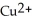 to flow freely between the two containers
to flow freely between the two containers
D) to allow for a balance of charge between the two chambers
A) to prevent any further migration of electrons through the wire
B) to allow for the build up of positively charged ions in one container and negatively charged ions in the other container
C) to allow the
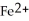 and the
and the 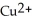 to flow freely between the two containers
to flow freely between the two containersD) to allow for a balance of charge between the two chambers

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
42
Chemical equations need to be balanced not only in terms of the number of atoms, but also by the charge. In other words, just as there should be the same number of atoms before and after the arrow of an equation, there should be the same charge. What set of coefficients is necessary to balance the following chemical equation? ____ 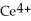 + ____ Cl⁻ → ____
+ ____ Cl⁻ → ____ 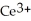 + ____
+ ____ 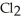
A) 1, 2, 1, 1
B) 2, 2, 2, 1
C) 1, 4, 1, 2
D) 3, 4, 3, 2
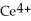 + ____ Cl⁻ → ____
+ ____ Cl⁻ → ____ 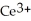 + ____
+ ____ 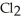
A) 1, 2, 1, 1
B) 2, 2, 2, 1
C) 1, 4, 1, 2
D) 3, 4, 3, 2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements best describes an anode?
A) the negatively charged electrode of a battery or electrochemical apparatus
B) the place where oxidation is taking place in a battery or electrochemical apparatus
C) the place from which electrons are flowing away from
D) all of the above
E) none of the above
A) the negatively charged electrode of a battery or electrochemical apparatus
B) the place where oxidation is taking place in a battery or electrochemical apparatus
C) the place from which electrons are flowing away from
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following describes an electrode?
A) Any material that conducts electrons into a media where electrochemical reactions are taking place.
B) Any material that conducts electrons out of a media where electrochemical reactions are taking place.
C) Any material that conducts electrons.
D) Any material that is undergoing an oxidation-reduction reaction in a battery.
E) either A or B
A) Any material that conducts electrons into a media where electrochemical reactions are taking place.
B) Any material that conducts electrons out of a media where electrochemical reactions are taking place.
C) Any material that conducts electrons.
D) Any material that is undergoing an oxidation-reduction reaction in a battery.
E) either A or B

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements best describes a cathode?
A) the negatively charged electrode of a battery or electrochemical apparatus
B) the place where oxidation is taking place in a battery or electrochemical apparatus
C) the place from which electrons are flowing away from
D) all of the above
E) none of the above
A) the negatively charged electrode of a battery or electrochemical apparatus
B) the place where oxidation is taking place in a battery or electrochemical apparatus
C) the place from which electrons are flowing away from
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
46
The following set of redox reactions takes place as shown. Container A Container B
Fe → Fe2+ + 2e- Cu2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) and a salt-bridge connecting the two containers, describe what happens.
A) A little bit of iron is converted into iron ions and a little bit of copper ion is converted into copper metal.
B) All of the iron is transformed into iron ions and all of the copper is transformed into copper ions.
C) All of the iron is transformed into iron ions and all of the copper ions are transformed into copper metal.
D) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper ions.
E) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper metal.
Fe → Fe2+ + 2e- Cu2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) and a salt-bridge connecting the two containers, describe what happens.
A) A little bit of iron is converted into iron ions and a little bit of copper ion is converted into copper metal.
B) All of the iron is transformed into iron ions and all of the copper is transformed into copper ions.
C) All of the iron is transformed into iron ions and all of the copper ions are transformed into copper metal.
D) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper ions.
E) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper metal.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
47
The following set of redox reactions takes place as shown. Container A Container B
Fe → Fe2+ + 2e- Cu 2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) and a salt-bridge connecting the two containers, which way do the positive ions in the salt-bridge flow?
A) There is no flow of positive ions in the salt-bridge
B) The positive ions flow from container A to container B.
C) The positive ions flow from container B to container A.
D) The positive ions flow back and forth between the two containers.
E) The ions only move in the containers they originate in.
Fe → Fe2+ + 2e- Cu 2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) and a salt-bridge connecting the two containers, which way do the positive ions in the salt-bridge flow?
A) There is no flow of positive ions in the salt-bridge
B) The positive ions flow from container A to container B.
C) The positive ions flow from container B to container A.
D) The positive ions flow back and forth between the two containers.
E) The ions only move in the containers they originate in.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
48
The following set of redox reactions takes place between iron dipped in a solution of copper ions. Fe → Fe2+ + 2e-
Cu2+ + 2e- → Cu
Which of the following describes what is happening on the surface of the iron?
A) The copper starts to plate out on iron.
B) The iron rusts in the water.
C) The iron starts to plate out on the copper.
D) The iron corrodes the copper.
E) none of the above
Cu2+ + 2e- → Cu
Which of the following describes what is happening on the surface of the iron?
A) The copper starts to plate out on iron.
B) The iron rusts in the water.
C) The iron starts to plate out on the copper.
D) The iron corrodes the copper.
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
49
The following set of redox reactions takes place as shown. Container A Container B
Fe→ Fe2+ + 2e- Cu2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB), describe what happens.
A) A little bit of iron is converted into iron ions and a little bit of copper ion is converted into copper metal.
B) All of the iron is transformed into iron ions and all of the copper is transformed into copper ions.
C) All of the iron is transformed into iron ions and all of the copper ions are transformed into copper metal.
D) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper ions.
E) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper metal.
Fe→ Fe2+ + 2e- Cu2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB), describe what happens.
A) A little bit of iron is converted into iron ions and a little bit of copper ion is converted into copper metal.
B) All of the iron is transformed into iron ions and all of the copper is transformed into copper ions.
C) All of the iron is transformed into iron ions and all of the copper ions are transformed into copper metal.
D) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper ions.
E) All of the iron ions are transformed into iron metal and all of the copper is transformed into copper metal.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
50
Why is it easier for the body to excrete a polar molecule than it is to excrete a nonpolar molecule? What chemistry does the body use to get rid of molecules it no longer needs?
A) It is easier for the body to excrete non-polar molecules because they dissolve in the oils of the skin and are washed away when we bathe.
B) Polar molecules are easier to excrete because of their greater solubility in water. Upon dissolving in water, they can be excreted through urine.
C) Polarity of the molecules has nothing to do with the ease of excretion. However, the body does metabolize and excrete smaller molecules more easily and passes them out through the colon.
D) Polar and non-polar molecules are excreted equally easily. The body is well adapted to deal with both forms of molecules via its metabolic processes.
A) It is easier for the body to excrete non-polar molecules because they dissolve in the oils of the skin and are washed away when we bathe.
B) Polar molecules are easier to excrete because of their greater solubility in water. Upon dissolving in water, they can be excreted through urine.
C) Polarity of the molecules has nothing to do with the ease of excretion. However, the body does metabolize and excrete smaller molecules more easily and passes them out through the colon.
D) Polar and non-polar molecules are excreted equally easily. The body is well adapted to deal with both forms of molecules via its metabolic processes.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
51
A battery operates by ________.
A) oxidation
B) reduction
C) both oxidation and reduction
D) none of the above
A) oxidation
B) reduction
C) both oxidation and reduction
D) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements about electrochemistry is not true?
A) It is the study of how electrical energy and chemical reactions are related.
B) It involves the use of a set of oxidation-reduction reactions to produce electrical current.
C) It involves the use of electrical current to produce an oxidation-reduction reaction.
D) It is the study of how electrons are transferred from one chemical compound to another.
E) All of the above are true.
A) It is the study of how electrical energy and chemical reactions are related.
B) It involves the use of a set of oxidation-reduction reactions to produce electrical current.
C) It involves the use of electrical current to produce an oxidation-reduction reaction.
D) It is the study of how electrons are transferred from one chemical compound to another.
E) All of the above are true.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
53
The following set of redox reactions takes place as shown. Container A Container B
Fe → Fe2+ + 2e- Cu+2 + 2e- → Cu
If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in containerA) and a piece of copper (in container B), which way do the electrons flow?
A) There is no continuous flow of electrons.
B) The electrons from from container A to container B.
C) The electrons flow from container B to container A.
D) The electrons flow back and forth between the two containers.
E) The electrons only move in the containers they originate in.
Fe → Fe2+ + 2e- Cu+2 + 2e- → Cu
If you had two containers filled with the ion solution described above with only a wire connecting a piece of iron (in containerA) and a piece of copper (in container B), which way do the electrons flow?
A) There is no continuous flow of electrons.
B) The electrons from from container A to container B.
C) The electrons flow from container B to container A.
D) The electrons flow back and forth between the two containers.
E) The electrons only move in the containers they originate in.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
54
The following set of redox reactions takes place as shown. Container A Container B
Fe → Fe2+ + 2e- Cu2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) and a salt-bridge connecting the two containers, which way do the electrons flow?
A) There is no continuous flow of electrons.
B) The electrons flow from container A to container B.
C) The electrons flow from container B to container A.
D) The electrons flow back and forth between the two containers.
E) The electrons only move in the containers they originate in.
Fe → Fe2+ + 2e- Cu2+ + 2e- → Cu
If you had two containers filled with the ion solution described above with a wire connecting a piece of iron (in containerA) and a piece of copper (in containerB) and a salt-bridge connecting the two containers, which way do the electrons flow?
A) There is no continuous flow of electrons.
B) The electrons flow from container A to container B.
C) The electrons flow from container B to container A.
D) The electrons flow back and forth between the two containers.
E) The electrons only move in the containers they originate in.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
55
What is the purpose of a salt-bridge?
A) to allow ions to migrate between solutions
B) to allow people to cross over salt water
C) to allow electrons to migrate through solutions
D) to allow salt to form in the oxidation-reduction reactions
E) none of the above
A) to allow ions to migrate between solutions
B) to allow people to cross over salt water
C) to allow electrons to migrate through solutions
D) to allow salt to form in the oxidation-reduction reactions
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
56
The zinc within a copper penny does not oxidize because ________.
A) zinc is an inert metal
B) it is not exposed to oxygen
C) of an impossible build up of charge
D) of a lack of water
A) zinc is an inert metal
B) it is not exposed to oxygen
C) of an impossible build up of charge
D) of a lack of water

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
57
The following set of redox reactions takes place when iron is dipped into a solution of copper ions. Fe → Fe2+ + 2e-
Cu2+ + 2e- → Cu
Which of the following describes what is happening with the electrons in the solution?
A) The iron metal is releasing electrons and they are traveling to the copper ions.
B) The copper is releasing electrons and they are traveling to the iron ions.
C) The iron ions are gaining electrons from the copper metal.
D) The copper ions are releasing electrons and they are traveling to the iron metal.
E) none of the above
Cu2+ + 2e- → Cu
Which of the following describes what is happening with the electrons in the solution?
A) The iron metal is releasing electrons and they are traveling to the copper ions.
B) The copper is releasing electrons and they are traveling to the iron ions.
C) The iron ions are gaining electrons from the copper metal.
D) The copper ions are releasing electrons and they are traveling to the iron metal.
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
58
Upon ingestion, grain alcohol, 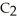
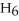 O, is metabolized into acetaldehyde,
O, is metabolized into acetaldehyde, 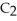
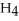 O, which is a toxic substance causing headaches as well as joint pains typical of a "hangover." Is the grain alcohol oxidized or reduced as it transforms into acetaldehyde?
O, which is a toxic substance causing headaches as well as joint pains typical of a "hangover." Is the grain alcohol oxidized or reduced as it transforms into acetaldehyde?
A) The grain alcohol is reduced.
B) The grain alcohol is oxidized.
C) Some of the grain alcohol is oxidized and some is reduced to other products.
D) The grain alcohol is neither oxidized nor reduced.
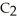
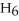 O, is metabolized into acetaldehyde,
O, is metabolized into acetaldehyde, 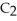
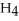 O, which is a toxic substance causing headaches as well as joint pains typical of a "hangover." Is the grain alcohol oxidized or reduced as it transforms into acetaldehyde?
O, which is a toxic substance causing headaches as well as joint pains typical of a "hangover." Is the grain alcohol oxidized or reduced as it transforms into acetaldehyde?A) The grain alcohol is reduced.
B) The grain alcohol is oxidized.
C) Some of the grain alcohol is oxidized and some is reduced to other products.
D) The grain alcohol is neither oxidized nor reduced.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
59
Glucose, 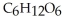 , is a simple sugar that the body metabolizes into two molecules of pyruvic acid,
, is a simple sugar that the body metabolizes into two molecules of pyruvic acid, 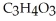 . Is the glucose oxidized or reduced as it transforms into pyruvic acid?
. Is the glucose oxidized or reduced as it transforms into pyruvic acid?
A) The glucose is oxidized.
B) The glucose is reduced.
C) Parts of the glucose molecule are oxidized while others are reduced.
D) Glucose is neither oxidized or reduced as it transforms into pyruvic acid.
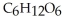 , is a simple sugar that the body metabolizes into two molecules of pyruvic acid,
, is a simple sugar that the body metabolizes into two molecules of pyruvic acid, 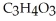 . Is the glucose oxidized or reduced as it transforms into pyruvic acid?
. Is the glucose oxidized or reduced as it transforms into pyruvic acid?A) The glucose is oxidized.
B) The glucose is reduced.
C) Parts of the glucose molecule are oxidized while others are reduced.
D) Glucose is neither oxidized or reduced as it transforms into pyruvic acid.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
60
In the oxidation-reduction reaction Mg(s) + 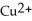 (aq) →
(aq) → 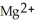 (aq) + Cu(s), which atom or ion is reduced? Which atom or ion is oxidized?
(aq) + Cu(s), which atom or ion is reduced? Which atom or ion is oxidized?
A) The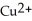 ion is oxidized as it gains electrons from the copper metal, Cu. The magnesium metal, Mg, is reduced as it loses electrons to form
ion is oxidized as it gains electrons from the copper metal, Cu. The magnesium metal, Mg, is reduced as it loses electrons to form 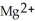 .
.
B) The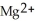 ion is reduced as it gains electrons from the copper metal, Cu. The
ion is reduced as it gains electrons from the copper metal, Cu. The 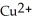 is oxidized as it loses electrons to the
is oxidized as it loses electrons to the 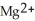 .
.
C) The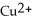 ion is reduced as it gains electrons to form copper metal, Cu. The magnesium metal, Mg, is oxidized as it loses electrons to form
ion is reduced as it gains electrons to form copper metal, Cu. The magnesium metal, Mg, is oxidized as it loses electrons to form 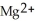 .
.
D) Since Mg goes from a solid to an aqueous solution and Cu goes from an aqueous solution to a solid, an oxidation-reduction reaction did not occur.
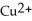 (aq) →
(aq) → 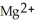 (aq) + Cu(s), which atom or ion is reduced? Which atom or ion is oxidized?
(aq) + Cu(s), which atom or ion is reduced? Which atom or ion is oxidized?A) The
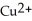 ion is oxidized as it gains electrons from the copper metal, Cu. The magnesium metal, Mg, is reduced as it loses electrons to form
ion is oxidized as it gains electrons from the copper metal, Cu. The magnesium metal, Mg, is reduced as it loses electrons to form 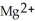 .
.B) The
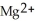 ion is reduced as it gains electrons from the copper metal, Cu. The
ion is reduced as it gains electrons from the copper metal, Cu. The 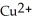 is oxidized as it loses electrons to the
is oxidized as it loses electrons to the 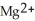 .
.C) The
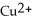 ion is reduced as it gains electrons to form copper metal, Cu. The magnesium metal, Mg, is oxidized as it loses electrons to form
ion is reduced as it gains electrons to form copper metal, Cu. The magnesium metal, Mg, is oxidized as it loses electrons to form 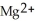 .
.D) Since Mg goes from a solid to an aqueous solution and Cu goes from an aqueous solution to a solid, an oxidation-reduction reaction did not occur.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
61
In a battery, the following two oxidation-reduction reactions are taking place: rxn A: Zn + 2 OH- → ZnO + H2O + 2e-
Rxn B: 2 MnO2 + H2O + 2e- → Mn2O3 + 2 OH-
What is undergoing oxidation?
A) Zn
B) ZnO
C) MnO2
D) H2O
E) OH-
Rxn B: 2 MnO2 + H2O + 2e- → Mn2O3 + 2 OH-
What is undergoing oxidation?
A) Zn
B) ZnO
C) MnO2
D) H2O
E) OH-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
62
Why is the anode of a battery indicated with negative (-) sign?
A) Because that is where electrons are being generated.
B) Because it is negative.
C) Because it is positive.
D) Because that is where electrons are being adsorbed.
E) It is just a convention so the batteries are put in the MP3 player correctly.
A) Because that is where electrons are being generated.
B) Because it is negative.
C) Because it is positive.
D) Because that is where electrons are being adsorbed.
E) It is just a convention so the batteries are put in the MP3 player correctly.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
63
Why might disposing of a lead-acid battery, nickel-cadmium battery or mercury battery in a landfill be a bad thing?
A) Cadmium, mercury, and lead are all toxic metals and could leak out of the landfill.
B) Cadmium, mercury, and lead are all very reactive and generate highly unstable compounds.
C) Cadmium, mercury, and lead are all radioactive and release radiation.
D) all of the above
E) none of the above
A) Cadmium, mercury, and lead are all toxic metals and could leak out of the landfill.
B) Cadmium, mercury, and lead are all very reactive and generate highly unstable compounds.
C) Cadmium, mercury, and lead are all radioactive and release radiation.
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
64
Zinc, Zn, is more easily oxidized than copper, Cu. Two strips of these metals are placed within separate salt solutions and then connected by a wire. Does an electric current pass through this wire?
A) Yes, as the zinc is oxidized in the salt solution, its released electrons pass through the wire producing an electric current.
B) No, an electric current does not pass through the wire because this would result in a prohibitive build up of charge.
C) Yes and No. The passage of an electric current depends on whether the salt solutions are sufficiently concentrated to cause the oxidation of the zinc metal. The answer is yes only if the salt solution concentration is greater than 1M.
D) There is insufficient information given to know. The passage of electric current depends on three things: the size of the plates, the concentration of the salt solution, and the gauge of the wire.
A) Yes, as the zinc is oxidized in the salt solution, its released electrons pass through the wire producing an electric current.
B) No, an electric current does not pass through the wire because this would result in a prohibitive build up of charge.
C) Yes and No. The passage of an electric current depends on whether the salt solutions are sufficiently concentrated to cause the oxidation of the zinc metal. The answer is yes only if the salt solution concentration is greater than 1M.
D) There is insufficient information given to know. The passage of electric current depends on three things: the size of the plates, the concentration of the salt solution, and the gauge of the wire.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
65
What is the primary difference between a fuel cell and a battery?
A) Fuel cells do not run down because they can be refueled; batteries run down and need to be recharged.
B) Batteries can be recharged, fuel cells cannot.
C) Batteries supply electricity; fuel cells supply heat.
D) Fuel cells oxidize to supply electricity, batteries reduce to supply electricity.
E) Fuel cells do not use metals as oxidants and reductants, batteries have a static reservoir of oxidant and reductant.
A) Fuel cells do not run down because they can be refueled; batteries run down and need to be recharged.
B) Batteries can be recharged, fuel cells cannot.
C) Batteries supply electricity; fuel cells supply heat.
D) Fuel cells oxidize to supply electricity, batteries reduce to supply electricity.
E) Fuel cells do not use metals as oxidants and reductants, batteries have a static reservoir of oxidant and reductant.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
66
Some car batteries require the periodic addition of water. Does adding the water increase or decrease the battery's ability to provide electric power to start the car?
A) It will decrease the battery's ability to provide electrical power because it will dilute the amount of ions in contact with the electrode.
B) It will increase the battery's ability to provide electrical power because it will decrease the number of ions in contact with the electrode.
C) It will decrease the battery's ability to provide electrical power because water increases the surface area of the electrode in contact with the solution.
D) It will increase the battery's ability to provide electrical power because of the positive effect of diluting the ionic solution.
A) It will decrease the battery's ability to provide electrical power because it will dilute the amount of ions in contact with the electrode.
B) It will increase the battery's ability to provide electrical power because it will decrease the number of ions in contact with the electrode.
C) It will decrease the battery's ability to provide electrical power because water increases the surface area of the electrode in contact with the solution.
D) It will increase the battery's ability to provide electrical power because of the positive effect of diluting the ionic solution.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
67
In a battery, the following two oxidation-reduction reactions are taking place: rxn A: Zn → Zn2+ + 2e-
Rxn B: 2 NH4+ + 2e- → 2 NH3 + H2
Which reaction is taking place at the anode?
A) rxn A
B) rxn B
C) Both reactions are happening at both the anode and cathode.
D) Both reactions are happening at the electrode.
E) The reaction takes place at the electrode, not the anode.
Rxn B: 2 NH4+ + 2e- → 2 NH3 + H2
Which reaction is taking place at the anode?
A) rxn A
B) rxn B
C) Both reactions are happening at both the anode and cathode.
D) Both reactions are happening at the electrode.
E) The reaction takes place at the electrode, not the anode.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
68
Why does a battery that has thick zinc walls last longer than one that has thin zinc walls?
A) Thick zinc walls prevent the battery from over heating.
B) Thicker zinc walls prevent electrons from being lost into the surrounding environment.
C) Thicker zinc walls last longer at holding in the battery acid.
D) The zinc walls are transformed into zinc ions as the battery provides electricity.
A) Thick zinc walls prevent the battery from over heating.
B) Thicker zinc walls prevent electrons from being lost into the surrounding environment.
C) Thicker zinc walls last longer at holding in the battery acid.
D) The zinc walls are transformed into zinc ions as the battery provides electricity.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
69
A fuel cell produces electrical energy from ________.
A) a fuel
B) nuclear power
C) internal batteries
D) electrons
A) a fuel
B) nuclear power
C) internal batteries
D) electrons

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
70
In a battery, the following oxidation-reduction reaction is taking place: Mn2O3 + ZnO → 2 MnO2 + Zn
What is undergoing reduction as written?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above
What is undergoing reduction as written?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
71
In a battery, the following oxidation-reduction reactions are taking place: Mn2O3 + ZnO → 2 MnO2 + Zn
What is being formed at the cathode?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above
What is being formed at the cathode?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
72
Why is the anode of a battery indicated with a negative (-) sign?
A) Electrons are attracted to the negative electrode.
B) This electrode is the source of negatively charged electrons.
C) Electrons move to this area to react with N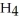 Cl in the battery.
Cl in the battery.
D) It indicates the electrode where the chemicals are reduced.
A) Electrons are attracted to the negative electrode.
B) This electrode is the source of negatively charged electrons.
C) Electrons move to this area to react with N
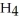 Cl in the battery.
Cl in the battery.D) It indicates the electrode where the chemicals are reduced.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
73
In a battery, the following two oxidation-reduction reactions are taking place: rxn A: Zn → Zn2+ + 2e-
Rxn B: 2 NH4+ + 2e- → 2 NH3 + H2
Which reaction is taking place at the cathode?
A) rxn A
B) rxn B
C) Both reactions are happening at both the anode and cathode.
D) Both reactions are happening are happening at the electrode.
E) The reaction takes place at the electrode, not the cathode.
Rxn B: 2 NH4+ + 2e- → 2 NH3 + H2
Which reaction is taking place at the cathode?
A) rxn A
B) rxn B
C) Both reactions are happening at both the anode and cathode.
D) Both reactions are happening are happening at the electrode.
E) The reaction takes place at the electrode, not the cathode.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
74
In a battery, if the following oxidation-reduction reactions takes place normally: Mn2O3 + ZnO → 2 MnO2 + Zn
What reaction would be taking place if you were charging the battery?
A) 2 MnO2 + Zn → Mn2O3 + ZnO
B) ZnO → Zn
C) Mn2O3 → MnO2
D) all of the above
E) none of the above
What reaction would be taking place if you were charging the battery?
A) 2 MnO2 + Zn → Mn2O3 + ZnO
B) ZnO → Zn
C) Mn2O3 → MnO2
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
75
Your car lights were left on while you were shopping, and now your car battery is dead. Has the pH of the battery fluid increased or decreased?
A) The pH of the battery fluid has increased.
B) The pH of the battery fluid has decreased.
C) The pH of the battery fluid does not change.
D) Since the lights were left on and the battery is dead, the pH of the battery fluid must be zero.
A) The pH of the battery fluid has increased.
B) The pH of the battery fluid has decreased.
C) The pH of the battery fluid does not change.
D) Since the lights were left on and the battery is dead, the pH of the battery fluid must be zero.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
76
In a battery, the following two oxidation-reduction reactions are taking place: rxn A: Zn + 2 OH- → ZnO + H2O + 2e-
Rxn B: 2 MnO2 + H2O + 2e- → Mn2O3 + 2 OH-
What is undergoing reduction?
A) Zn
B) ZnO
C) MnO2
D) H2O
E) OH-
Rxn B: 2 MnO2 + H2O + 2e- → Mn2O3 + 2 OH-
What is undergoing reduction?
A) Zn
B) ZnO
C) MnO2
D) H2O
E) OH-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
77
In a battery, the following oxidation-reduction reactions are taking place: Mn2O3 + ZnO → 2 MnO2 + Zn
What is being formed at the anode?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above
What is being formed at the anode?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
78
In one type of fuel cell the following oxidation-reduction reactions are taking place: 2 H2 + O2 → 2 H2O
What is the fuel ?
A) H2
B) O2
C) H2O
D) all of the above
E) none of the above
What is the fuel ?
A) H2
B) O2
C) H2O
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
79
In a battery, the following oxidation-reduction reactions are taking place: Mn2O3 + ZnO → 2 MnO2 + Zn
What is undergoing an oxidation as written?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above
What is undergoing an oxidation as written?
A) Zn
B) ZnO
C) MnO2
D) Mn2O3
E) none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
80
Zinc, Zn, is more easily oxidized than copper, Cu. What happens the moment after metal strips of these metals come into contact with each other?
A) The zinc metal immediately begins depositing electrons onto the surface of the copper, thus coating the copper with a white powder of zinc oxide, ZnO.
B) The zinc metal begins to turn a greenish color indicating the presence of the formation of cupric oxide, CuO.
C) The surface of the copper appears moist and cloudy as the zinc metal oxidizes and gets dissolved into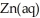 ions.
ions.
D) Nothing happens. An immediate build up of charge in either material prevents continued oxidation-reduction from occurring.
A) The zinc metal immediately begins depositing electrons onto the surface of the copper, thus coating the copper with a white powder of zinc oxide, ZnO.
B) The zinc metal begins to turn a greenish color indicating the presence of the formation of cupric oxide, CuO.
C) The surface of the copper appears moist and cloudy as the zinc metal oxidizes and gets dissolved into
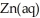 ions.
ions.D) Nothing happens. An immediate build up of charge in either material prevents continued oxidation-reduction from occurring.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck


