Deck 11: Using Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/106
Play
Full screen (f)
Deck 11: Using Energy
1
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the change of internal energy over the full cycle 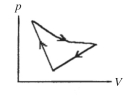
A) is positive.
B) is negative.
C) is zero.
D) cannot be determined from the information given.

A) is positive.
B) is negative.
C) is zero.
D) cannot be determined from the information given.
C
2
The temperature in your classroom is closest to
A) 68 K.
B) 68°C.
C) 50°C.
D) 295 K.
A) 68 K.
B) 68°C.
C) 50°C.
D) 295 K.
D
3
A temperature change of 20 C° corresponds to a Fahrenheit temperature change of
A) 68 F°.
B) 11 F°.
C) 36 F°.
D) 18 F°.
A) 68 F°.
B) 11 F°.
C) 36 F°.
D) 18 F°.
C
4
An ideal gas undergoes an isothermal expansion. During this process, its entropy
A) decreases.
B) remains unchanged.
C) increases.
D) cannot be predicted from the data given.
A) decreases.
B) remains unchanged.
C) increases.
D) cannot be predicted from the data given.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
5
When water at 0°C freezes, the entropy of the water
A) increases.
B) decreases.
C) remains constant.
D) could either increase or decrease; it depends on other factors.
A) increases.
B) decreases.
C) remains constant.
D) could either increase or decrease; it depends on other factors.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a false statement?
A) Entropy is a quantitative measure of disorder.
B) The total entropy change in one cycle of a Carnot engine is zero.
C) The entropy of an isolated system must be conserved (it remains constant).
D) Entropy can be measured in units of J/K.
A) Entropy is a quantitative measure of disorder.
B) The total entropy change in one cycle of a Carnot engine is zero.
C) The entropy of an isolated system must be conserved (it remains constant).
D) Entropy can be measured in units of J/K.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
7
A certain gas is compressed adiabatically. The amount of work done on the gas is 800 J. What is the change in the internal (thermal) energy of the gas?
A) 800 J
B) -800 J
C) 400 J
D) 0 J
E) More information is needed to answer this question.
A) 800 J
B) -800 J
C) 400 J
D) 0 J
E) More information is needed to answer this question.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
8
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a counter-clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents 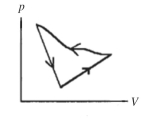
A) the heat that flows out of the gas.
B) the work done on the gas.
C) the heat added to the gas.
D) the work done by the gas.

A) the heat that flows out of the gas.
B) the work done on the gas.
C) the heat added to the gas.
D) the work done by the gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following is a true statement?
A) The second law of thermodynamics is a consequence of the first law of thermodynamics.
B) It is possible for heat to flow spontaneously from a hot body to a cold one or from a cold one to a hot one, depending on whether or not the process is reversible or irreversible.
C) It is not possible to convert work entirely into heat.
D) It is impossible to transfer heat from a cooler to a hotter body.
E) All of these statements are false.
A) The second law of thermodynamics is a consequence of the first law of thermodynamics.
B) It is possible for heat to flow spontaneously from a hot body to a cold one or from a cold one to a hot one, depending on whether or not the process is reversible or irreversible.
C) It is not possible to convert work entirely into heat.
D) It is impossible to transfer heat from a cooler to a hotter body.
E) All of these statements are false.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
10
If the efficiency of a Carnot engine were to be 100%, the heat sink would have to be
A) at absolute zero.
B) at 0°C.
C) at 100°C.
D) infinitely hot.
A) at absolute zero.
B) at 0°C.
C) at 100°C.
D) infinitely hot.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
11
At what, if any, temperature are the numerical readings on the Fahrenheit and Celsius scales the same?
A) -30°
B) -40°
C) -50°
D) -60°
E) They can never read the same because they are based on different zeroes.
A) -30°
B) -40°
C) -50°
D) -60°
E) They can never read the same because they are based on different zeroes.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
12
An important feature of the Carnot cycle is that
A) its efficiency can be 100%.
B) its efficiency depends only on the absolute temperature of the hot reservoir used.
C) its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used, and on nothing else.
D) it is an example of an irreversible process that can be analyzed exactly without approximations.
E) no engine can be more efficient than a Carnot engine operating between the same two temperatures.
A) its efficiency can be 100%.
B) its efficiency depends only on the absolute temperature of the hot reservoir used.
C) its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used, and on nothing else.
D) it is an example of an irreversible process that can be analyzed exactly without approximations.
E) no engine can be more efficient than a Carnot engine operating between the same two temperatures.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
13
The temperature changes from 35°F during the night to 75°F during the day. What is the temperature change on the Celsius scale?
A) 72 C°
B) 40 C°
C) 32 C°
D) 22 C°
A) 72 C°
B) 40 C°
C) 32 C°
D) 22 C°

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
14
Express a body temperature 98.6°F in Celsius degrees.
A) 37.0°C
B) 45.5°C
C) 66.6°C
D) 72.6°C
A) 37.0°C
B) 45.5°C
C) 66.6°C
D) 72.6°C

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
15
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents 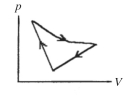
A) the heat that flows out of the gas.
B) the work done on the gas.
C) the heat added to the gas.
D) the work done by the gas.

A) the heat that flows out of the gas.
B) the work done on the gas.
C) the heat added to the gas.
D) the work done by the gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
16
A Carnot cycle consists of
A) two adiabats and two isobars.
B) two isobars and two isotherms.
C) four isotherms.
D) two adiabats and two isotherms.
E) four adiabats.
A) two adiabats and two isobars.
B) two isobars and two isotherms.
C) four isotherms.
D) two adiabats and two isotherms.
E) four adiabats.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
17
Which two temperature changes are equivalent?
A) 1 K = 1 F°
B) 1 F° = 1 C°
C) 1 C° = 1 K
D) none of the above
A) 1 K = 1 F°
B) 1 F° = 1 C°
C) 1 C° = 1 K
D) none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
18
Express -40°C in °F.
A) -72°F
B) -54°F
C) -40°F
D) 4.4°F
A) -72°F
B) -54°F
C) -40°F
D) 4.4°F

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
19
A 10-L flask and a 1-L flask each contain two moles of ideal diatomic gas (but not the same gas) at 25°C. Which of the following statements about these gases must be true? (There could be more than one correct choice.)
A) The internal (thermal) energy of the gas in both flasks is the same.
B) The internal (thermal) energy of the gas in the larger flask is greater than the internal (thermal) energy of the gas in the smaller flask.
C) The internal (thermal) energy of the gas in the smaller flask is greater than the internal (thermal) energy of the gas in the larger flask.
D) The molecules in the larger flask have the same root-mean-square speed as those in the smaller flask.
E) The molecules in the smaller flask have the same average kinetic energy per molecule as those in the larger flask.
A) The internal (thermal) energy of the gas in both flasks is the same.
B) The internal (thermal) energy of the gas in the larger flask is greater than the internal (thermal) energy of the gas in the smaller flask.
C) The internal (thermal) energy of the gas in the smaller flask is greater than the internal (thermal) energy of the gas in the larger flask.
D) The molecules in the larger flask have the same root-mean-square speed as those in the smaller flask.
E) The molecules in the smaller flask have the same average kinetic energy per molecule as those in the larger flask.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
20
The second law of thermodynamics leads us to conclude that
A) the total energy of the universe is constant.
B) disorder in the universe is increasing with the passage of time.
C) it is theoretically possible to convert heat into work with 100% efficiency.
D) the average temperature of the universe is increasing with the passage of time.
E) the entropy of the universe remains constant.
A) the total energy of the universe is constant.
B) disorder in the universe is increasing with the passage of time.
C) it is theoretically possible to convert heat into work with 100% efficiency.
D) the average temperature of the universe is increasing with the passage of time.
E) the entropy of the universe remains constant.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
21
In an isochoric process, the internal (thermal) energy of a gas decreases by 50 J. How much work is done by the gas during this process?
A) 0 J
B) 50 J
C) -50 J
D) 25 J
E) -25 J
A) 0 J
B) 50 J
C) -50 J
D) 25 J
E) -25 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
22
A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 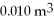 to
to 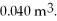 The final pressure is
The final pressure is 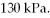 What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
A) 0.0 kJ
B) 3.6 kJ
C) 7.2 kJ
D) -3.6 kJ
E) -7.2 kJ
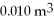 to
to 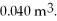 The final pressure is
The final pressure is 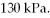 What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)A) 0.0 kJ
B) 3.6 kJ
C) 7.2 kJ
D) -3.6 kJ
E) -7.2 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
23
An external heat source supplies heat to a system at a rate of 187 W as the system does work at a rate of 131 W. At what rate is the internal (thermal) energy of the system changing?
A) -56 W
B) 320 W
C) 56 W
D) 190 W
E) -320 W
A) -56 W
B) 320 W
C) 56 W
D) 190 W
E) -320 W

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
24
The weather outside is frightful. The temperature is -22°F. What is the corresponding temperature in the Celsius scale?
A) -35°C
B) -30°C
C) -22°C
D) -20°C
E) -12°C
A) -35°C
B) -30°C
C) -22°C
D) -20°C
E) -12°C

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
25
During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the work done by the gas in the process?
A) 0 J
B) 5.0 J
C) -5.0 J
D) -10 J
A) 0 J
B) 5.0 J
C) -5.0 J
D) -10 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
26
A person consumes a snack containing 14 food calories (14 kcal). What is the power this food produces if it is to be "burned off" due to exercise in 6 hours? (1 cal = 4.186 J)
A) 2.7 W
B) 9763 W
C) 0.6 W
D) 0.0027 W
A) 2.7 W
B) 9763 W
C) 0.6 W
D) 0.0027 W

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
27
An ideal gas undergoes an adiabatic process while doing 25 J of work. What is the change in the internal (thermal) energy of the gas?
A) 0 J
B) 25 J
C) -25 J
D) 50 J
E) -50 J
A) 0 J
B) 25 J
C) -25 J
D) 50 J
E) -50 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
28
Oxygen condenses into a liquid at approximately 90 K. What temperature, in degrees Fahrenheit, does this correspond to?
A) -193°F
B) -217°F
C) -265°F
D) -297°F
A) -193°F
B) -217°F
C) -265°F
D) -297°F

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
29
The work done on an ideal gas system in an isothermal process is -400 J. What is the change in internal (thermal) energy of the gas?
A) 0 J
B) -400 J
C) 400 J
D) 200 J
A) 0 J
B) -400 J
C) 400 J
D) 200 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
30
During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the change in internal (thermal) energy of the gas?
A) 0 J
B) 2.5 J
C) 5.0 J
D) 10 J
A) 0 J
B) 2.5 J
C) 5.0 J
D) 10 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
31
The figure shows a pV diagram for a gas going through a cycle from A to B to C and back to A. From point A to point B, the gas absorbs 50 J of heat and finds its internal (thermal) energy has increased by 20 J. Going from B to C, the internal (thermal) energy decreases by 5.0 J.
(a) How much work was done by the gas from A to B?
(b) How much heat was absorbed by the gas from B to C?
(c) How much work was done by the gas going from B to C?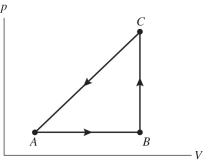
(a) How much work was done by the gas from A to B?
(b) How much heat was absorbed by the gas from B to C?
(c) How much work was done by the gas going from B to C?


Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
32
During an isochoric process, the internal (thermal) energy of a gas decreases by 50 J. How much heat is added to the gas during this process?
A) 0 J
B) 50 J
C) -50 J
D) 25 J
E) -25 J
A) 0 J
B) 50 J
C) -50 J
D) 25 J
E) -25 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
33
The gas in a perfectly insulated but flexible container does work at a rate of 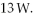 At what rate is the internal (thermal) energy of the gas changing?
At what rate is the internal (thermal) energy of the gas changing?
A) -13 W
B) 13 W
C) 0 W
D) 6.5 W
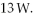 At what rate is the internal (thermal) energy of the gas changing?
At what rate is the internal (thermal) energy of the gas changing?A) -13 W
B) 13 W
C) 0 W
D) 6.5 W

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
34
A fixed amount of an ideal monatomic gas is maintained at constant volume as it is cooled by 50 K. This feat is accomplished by removing 400 J of energy from the gas. How much work is done by the gas during this process?
A) 0 J
B) 400 J
C) -400 J
D) -200 J
E) 200 J
A) 0 J
B) 400 J
C) -400 J
D) -200 J
E) 200 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
35
At room temperature, a typical person loses energy to the surroundings at the rate of 62 W. If this energy loss has to be made up by an equivalent food intake, how many kilocalories (food calories) does this person need to consume every day just to make up this heat loss? (1 cal = 4.186 J)
A) 1000 kcal
B) 1100 kcal
C) 1300 kcal
D) 1500 kcal
E) 1600 kcal
A) 1000 kcal
B) 1100 kcal
C) 1300 kcal
D) 1500 kcal
E) 1600 kcal

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
36
A person running in place on an exercise machine for 10 min uses up 17 kcal (food calories). Another person exercises by repeatedly lifting two 2.5-kg weights a distance of 50 cm. How many repetitions of this exercise are equivalent to 10 minutes of running in place? Assume that the person uses negligible energy in letting down the weights after each lift. (1 cal = 4.186 J)
A) 730
B) 1450
C) 1500
D) 2200
E) 2900
A) 730
B) 1450
C) 1500
D) 2200
E) 2900

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
37
Platinum melts at 3215°F. What is the corresponding temperature in the Kelvin scale?
A) 2041 K
B) 2135 K
C) 2207 K
D) 2296 K
E) 3215 K
A) 2041 K
B) 2135 K
C) 2207 K
D) 2296 K
E) 3215 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
38
What is absolute zero on the (a) Celsius scale and (b) on the Fahrenheit scale?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
39
In an adiabatic compression, 200 J of work is done on a gas. What is the change in internal (thermal) energy of the gas during this compression?
A) 0 J
B) 100 J
C) 200 J
D) -200 J
A) 0 J
B) 100 J
C) 200 J
D) -200 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
40
Nitrogen boils at -196°C. What is the corresponding temperature in the Fahrenheit scale?
A) -315°F
B) -196°F
C) -346°F
D) -290°F
E) -321°F
A) -315°F
B) -196°F
C) -346°F
D) -290°F
E) -321°F

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
41
A cylinder contains 8.8 moles of ideal gas, initially at a temperature of 126°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 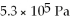 on the gas. The gas is cooled until its temperature has decreased to
on the gas. The gas is cooled until its temperature has decreased to 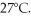 For the gas
For the gas 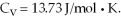 The ideal gas constant is R = 8.314 J/mol ∙ K. For this process, calculate:
The ideal gas constant is R = 8.314 J/mol ∙ K. For this process, calculate:
(a) the work done by gas
(b) the net change in the internal (thermal) energy of the gas
(c) the heat transferred to the gas.
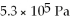 on the gas. The gas is cooled until its temperature has decreased to
on the gas. The gas is cooled until its temperature has decreased to 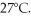 For the gas
For the gas 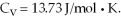 The ideal gas constant is R = 8.314 J/mol ∙ K. For this process, calculate:
The ideal gas constant is R = 8.314 J/mol ∙ K. For this process, calculate:(a) the work done by gas
(b) the net change in the internal (thermal) energy of the gas
(c) the heat transferred to the gas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
42
A heat engine absorbs 64 kcal of heat each cycle and exhausts 42 kcal.
(a) What is the efficiency of this engine?
(b) How much work does this engine do per cycle?
(a) What is the efficiency of this engine?
(b) How much work does this engine do per cycle?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
43
An athlete doing push-ups performs 650 kJ of work and loses 425 kJ of heat. What is the change in the internal (thermal) energy of the athlete?
A) -225 kJ
B) -1075 kJ
C) 1075 kJ
D) 225 kJ
E) 276 kJ
A) -225 kJ
B) -1075 kJ
C) 1075 kJ
D) 225 kJ
E) 276 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
44
A 40.0-L container is divided into two equal parts by a rubber membrane. One half of the container has 1.50 moles of an ideal monatomic gas at 250 K, and the other half is a vacuum. The container is well insulated, so there is no exchange of heat with the surroundings. The membrane breaks, and eventually the gas reaches a new equilibrium condition occupying the entire volume. What is the final temperature of the gas?
A) 100 K
B) 125 K
C) 157 K
D) 180 K
E) 250 K
A) 100 K
B) 125 K
C) 157 K
D) 180 K
E) 250 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
45
A sealed rigitd tank contains 30 moles of an ideal gas, at an initial temperature of 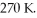 The pressure of the gas is increased until the final pressure equals 1.40 times the initial pressure. The heat capacity at constant pressure of the gas is
The pressure of the gas is increased until the final pressure equals 1.40 times the initial pressure. The heat capacity at constant pressure of the gas is 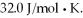 What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
A) 77 kJ
B) 100 kJ
C) 130 kJ
D) -50 kJ
E) -23 kJ
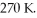 The pressure of the gas is increased until the final pressure equals 1.40 times the initial pressure. The heat capacity at constant pressure of the gas is
The pressure of the gas is increased until the final pressure equals 1.40 times the initial pressure. The heat capacity at constant pressure of the gas is 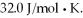 What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)A) 77 kJ
B) 100 kJ
C) 130 kJ
D) -50 kJ
E) -23 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
46
A cylinder contains 13 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equal 0.70 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol ∙ K. What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
A) -28 kJ
B) -38 kJ
C) 28 kJ
D) 38 kJ
E) -9.7 kJ
A) -28 kJ
B) -38 kJ
C) 28 kJ
D) 38 kJ
E) -9.7 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
47
The figure shows a pV diagram of a gas for a complete cycle. During part bc of the cycle, 1190 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 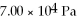 as its volume increases from
as its volume increases from 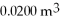 to
to 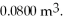 Calculate the change in internal (thermal) energy of the system during part bc of the cycle. If the change is nonzero, be sure to indicate whether the change is positive or negative.
Calculate the change in internal (thermal) energy of the system during part bc of the cycle. If the change is nonzero, be sure to indicate whether the change is positive or negative. 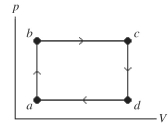
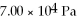 as its volume increases from
as its volume increases from 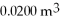 to
to 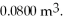 Calculate the change in internal (thermal) energy of the system during part bc of the cycle. If the change is nonzero, be sure to indicate whether the change is positive or negative.
Calculate the change in internal (thermal) energy of the system during part bc of the cycle. If the change is nonzero, be sure to indicate whether the change is positive or negative. 

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
48
An expandable container holds 2.30 mole of helium, He, gas with an initial pressure of 770 kPa and an initial volume of 2.10 L. The gas expands isothermally to a final pressure of 350 kPa. How much heat is gained by the gas during this process? (R = 8.31 J/mol ∙ K)
A) 1280 J
B) 792 J
C) 685 J
D) 1370 J
E) 1700 J
A) 1280 J
B) 792 J
C) 685 J
D) 1370 J
E) 1700 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
49
A cylinder contains 10 moles of an ideal gas at a temperature of 300 K. The gas is compressed at constant pressure until the final volume equals 0.77 times the initial volume. The molar heat capacity at constant volume of the gas is 24.0 J/mol ∙ K and R = 8.31 J/mol ∙ K . How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
A) -22 kJ
B) -17 kJ
C) 22 kJ
D) 17 kJ
E) -5.7 kJ
A) -22 kJ
B) -17 kJ
C) 22 kJ
D) 17 kJ
E) -5.7 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
50
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. The heat gained by the gas in process a→b is 546 J, while in process 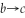 the gas loses 62.0 J of heat. In process a→b the gas performs
the gas loses 62.0 J of heat. In process a→b the gas performs 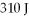 of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a?
of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a? 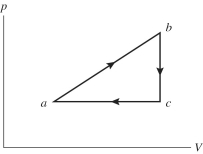
A) -397 J
B) -62 J
C) 223 J
D) 18 J
E) -236 J
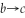 the gas loses 62.0 J of heat. In process a→b the gas performs
the gas loses 62.0 J of heat. In process a→b the gas performs 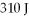 of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a?
of work, while in process c→a 223 J of work is done on the gas. How much heat is gained by the gas in process c→a? 
A) -397 J
B) -62 J
C) 223 J
D) 18 J
E) -236 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
51
A heat engine receives 7000 J of heat and loses 3000 J in each cycle. What is the efficiency of this engine?
A) 57%
B) 30%
C) 70%
D) 43%
A) 57%
B) 30%
C) 70%
D) 43%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
52
An expansion process on an ideal diatomic ideal gas for which CV = 5/2 R has a linear path between the initial and final coordinates on a pV diagram. The coordinates of the initial state are: the pressure is 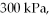 the volume is
the volume is 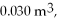 and the temperature is
and the temperature is 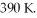 The final pressure is
The final pressure is 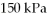 and the final temperature is
and the final temperature is 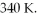 What is the change in the internal (thermal) energy of the gas, during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas, during this process? (R = 8.31 J/mol ∙ K)
A) -2900 J
B) -1700 J
C) 2900 J
D) 1700 J
E) 0 J
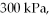 the volume is
the volume is 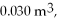 and the temperature is
and the temperature is 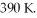 The final pressure is
The final pressure is 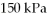 and the final temperature is
and the final temperature is 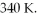 What is the change in the internal (thermal) energy of the gas, during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas, during this process? (R = 8.31 J/mol ∙ K)A) -2900 J
B) -1700 J
C) 2900 J
D) 1700 J
E) 0 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
53
A fluid in an insulated, flexible bottle is heated by a high resistance wire and expands. If 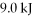 of heat is applied to the system and it does
of heat is applied to the system and it does 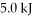 of work, how much does the internal (thermal) energy of the fluid change?
of work, how much does the internal (thermal) energy of the fluid change?
A) 4.0 kJ
B) 14 kJ
C) -4.0 kJ
D) 45 kJ
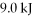 of heat is applied to the system and it does
of heat is applied to the system and it does 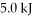 of work, how much does the internal (thermal) energy of the fluid change?
of work, how much does the internal (thermal) energy of the fluid change?A) 4.0 kJ
B) 14 kJ
C) -4.0 kJ
D) 45 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
54
A heat engine has an efficiency of 35.0% and receives 150 J of heat per cycle.
(a) How much work does it do in each cycle?
(b) How much heat does it "waste" in each cycle?
(a) How much work does it do in each cycle?
(b) How much heat does it "waste" in each cycle?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
55
A compression, at a constant pressure of 140 kPa, is performed on 4.0 moles of an ideal monatomic gas for which CV = 3/2 R. The compression reduces the volume of the gas from 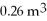 to
to 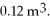 What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
A) -29 kJ
B) -49 kJ
C) 29 kJ
D) 49 kJ
E) 0 kJ
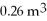 to
to 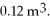 What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)
What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ∙ K)A) -29 kJ
B) -49 kJ
C) 29 kJ
D) 49 kJ
E) 0 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
56
A heat engine with an efficiency of 30% performs 2500 J of work. How much heat is discharged to the lower temperature reservoir?
A) 5800 J
B) 8300 J
C) 750 J
D) 1400 J
E) 7100 J
A) 5800 J
B) 8300 J
C) 750 J
D) 1400 J
E) 7100 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
57
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In the figure, Pa = Pc = 240 kPa, Vb = Vc = 40 L, Va = 15 L, and Pb = 400 kPa. How much heat is gained by the gas in this a→b→c→a process? 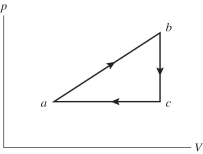
A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J

A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
58
A certain heat engine extracts 1.30 kJ of heat from a hot temperature reservoir and discharges 0.70 kJ of heat to a cold temperature reservoir. What is the efficiency of this engine?
A) 46%
B) 54%
C) 86%
D) 27%
E) 13%
A) 46%
B) 54%
C) 86%
D) 27%
E) 13%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
59
A cylinder contains 1.50 moles of an ideal monatomic gas that is initially at a temperature of 317 K. If the gas gains 2730 J of heat and performs 780 J of work, what is its final temperature? (R = 8.31 J/mol ∙ K)
A) 359 K
B) 421 K
C) 526 K
D) 687 K
E) 756 K
A) 359 K
B) 421 K
C) 526 K
D) 687 K
E) 756 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
60
A sealed rigid tank contains 29 moles of an ideal gas, at an initial temperature of 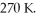 The pressure of the gas is increased until the final pressure equals 1.90 times the initial pressure. The heat capacity at constant pressure of the gas is
The pressure of the gas is increased until the final pressure equals 1.90 times the initial pressure. The heat capacity at constant pressure of the gas is 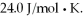 How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
A) 110 kJ
B) 170 kJ
C) 230 kJ
D) -52 kJ
E) 7.0 kJ
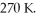 The pressure of the gas is increased until the final pressure equals 1.90 times the initial pressure. The heat capacity at constant pressure of the gas is
The pressure of the gas is increased until the final pressure equals 1.90 times the initial pressure. The heat capacity at constant pressure of the gas is 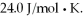 How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)A) 110 kJ
B) 170 kJ
C) 230 kJ
D) -52 kJ
E) 7.0 kJ

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
61
The figure shows a pV diagram for a cycle of a heat engine for which QH = 59 J. What is the thermal efficiency of the engine? 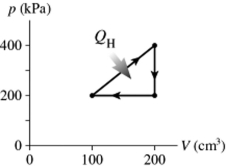
A) 17%
B) 34%
C) 8.5%
D) 14%

A) 17%
B) 34%
C) 8.5%
D) 14%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
62
An ideal Carnot engine extracts 529 J of heat from a high-temperature reservoir during each cycle, and rejects 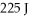 of heat to a low-temperature reservoir during the same cycle. What is the efficiency of the engine?
of heat to a low-temperature reservoir during the same cycle. What is the efficiency of the engine?
A) 0.57
B) 1.35
C) 2.35
D) 0.7
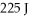 of heat to a low-temperature reservoir during the same cycle. What is the efficiency of the engine?
of heat to a low-temperature reservoir during the same cycle. What is the efficiency of the engine?A) 0.57
B) 1.35
C) 2.35
D) 0.7

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
63
An ideal Carnot heat engine operates between 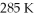 and
and 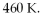 What is its efficiency?
What is its efficiency?
A) 0.38
B) 0.62
C) 0.61
D) 1.61
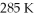 and
and 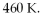 What is its efficiency?
What is its efficiency?A) 0.38
B) 0.62
C) 0.61
D) 1.61

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
64
A heat engine has an efficiency of 31.4% and receives 8.72 kJ of heat per cycle.
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
65
During each cycle, a heat engine takes in 4.0 J of heat, does 1.0 J of work, and expels 3.0 J of heat. What is its efficiency?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
66
A nuclear power plant has an actual efficiency of 33%. If 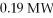 of energy are released from fission, how much electric power does the power plant produce?
of energy are released from fission, how much electric power does the power plant produce?
A) 0.063 MW
B) 6.3 MW
C) 25 MW
D) 0.25 MW
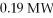 of energy are released from fission, how much electric power does the power plant produce?
of energy are released from fission, how much electric power does the power plant produce?A) 0.063 MW
B) 6.3 MW
C) 25 MW
D) 0.25 MW

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
67
An ideal Carnot heat engine operates between reservoirs at 1740 K and 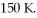 In each cycle, 260 J of heat energy is rejected to the low temperature reservoir. In each cycle, how much mechanical work W is performed by the engine?
In each cycle, 260 J of heat energy is rejected to the low temperature reservoir. In each cycle, how much mechanical work W is performed by the engine?
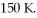 In each cycle, 260 J of heat energy is rejected to the low temperature reservoir. In each cycle, how much mechanical work W is performed by the engine?
In each cycle, 260 J of heat energy is rejected to the low temperature reservoir. In each cycle, how much mechanical work W is performed by the engine?
Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
68
A gas follows the pV trajectory shown in Figure 16.2. How much work is done per cycle by the gas if The gas in a heat engine follows the cycle shown in the pV diagram. How much work does this engine do each cycle if p0 = 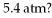
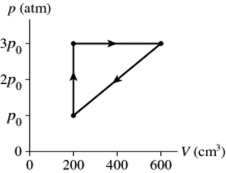
A) 220 J
B) 440 J
C) 870 J
D) 1100 J
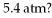

A) 220 J
B) 440 J
C) 870 J
D) 1100 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
69
A certain automobile engine takes in 4.00 kJ of heat and performs 1.10 kJ of mechanical work in each cycle.
(a) Calculate the engine's efficiency.
(b) How much heat is "wasted" in each cycle?
(a) Calculate the engine's efficiency.
(b) How much heat is "wasted" in each cycle?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
70
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day, and the heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C.
(a) What is the overall efficiency of the plant for generating electric power?
(b) How much thermal energy is exhausted each day?
(c) Using the same heat reservoirs, what is the maximum possible efficiency for a heat engine?
(a) What is the overall efficiency of the plant for generating electric power?
(b) How much thermal energy is exhausted each day?
(c) Using the same heat reservoirs, what is the maximum possible efficiency for a heat engine?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
71
An ideal Carnot heat engine has an efficiency of 0.600. If it operates between a deep lake with a constant temperature of 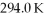 and a hot reservoir, what is the temperature of the hot reservoir?
and a hot reservoir, what is the temperature of the hot reservoir?
A) 735 K
B) 490 K
C) 470 K
D) 784 K
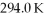 and a hot reservoir, what is the temperature of the hot reservoir?
and a hot reservoir, what is the temperature of the hot reservoir?A) 735 K
B) 490 K
C) 470 K
D) 784 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
72
An ideal Carnot engine has an efficiency of 83.0% and performs 4500 J of work every cycle. How much energy is discharged to the lower temperature reservoir every cycle?
A) 920 J
B) 830 J
C) 740 J
D) 3700 J
E) 5400 J
A) 920 J
B) 830 J
C) 740 J
D) 3700 J
E) 5400 J

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
73
Two ideal Carnot heat engines have the same efficiency. One operates between 5.0 × 102 K and 3.0 × 102 K, and the other between 4.0 × 102 K and some lower temperature. What is the lower temperature?
A) 200 K
B) 220 K
C) 240 K
D) 260 K
E) 280 K
A) 200 K
B) 220 K
C) 240 K
D) 260 K
E) 280 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
74
A heat engine having the maximum possible efficiency has an efficiency of 25% when operating between two heat reservoirs. If the temperature of the cold reservoir is 300 K, what is the temperature of the hot reservoir?
A) 350 K
B) 375 K
C) 400 K
D) 450 K
E) 500 K
A) 350 K
B) 375 K
C) 400 K
D) 450 K
E) 500 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
75
A heat engine having the maximum possible efficiency has an efficiency of 35.0% when operating between two heat reservoirs. If the temperature of the hot reservoir is 700 K, what is the temperature of the cold reservoir?
A) 200 K
B) 245 K
C) 350 K
D) 455 K
E) 600 K
A) 200 K
B) 245 K
C) 350 K
D) 455 K
E) 600 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
76
One of the most efficient engines built so far has the following characteristics: The combustion chamber temperature is 1900°C, the exhaust temperature = 430°C, 7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour. (1 cal = 4.186 J)
(a) What is the actual efficiency of this engine?
(b) What is the power output of this engine?
(c) What would be the maximum possible efficiency for an engine using the same temperature extremes?
(a) What is the actual efficiency of this engine?
(b) What is the power output of this engine?
(c) What would be the maximum possible efficiency for an engine using the same temperature extremes?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
77
A heat engine absorbs 85.6 kJ of heat each cycle and exhausts 61.8 kJ.
(a) What is the efficiency of the engine?
(b) How much work does it do each cycle?
(a) What is the efficiency of the engine?
(b) How much work does it do each cycle?

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
78
An ideal Carnot engine is operated between a hot and a cold reservoir. The temperature difference between the two reservoirs is 284°C. If the efficiency of this ideal engine is 24.0%, find the temperature of the cold reservoir in degrees Celsius.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
79
For a certain ideal Carnot engine, the hot reservoir is 35 C° higher than the cold reservoir. If this engine is to have an efficiency of 20%, what must be the temperature of the hot reservoir?
A) 70.0 K
B) 140 K
C) 175 K
D) 210 K
E) 245 K
A) 70.0 K
B) 140 K
C) 175 K
D) 210 K
E) 245 K

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
80
The ocean thermal energy conversion project uses the surface water near tropical islands with a temperature of 20°C as the hot temperature reservoir, and the water at some depth, with a temperature of 5.0°C, as the cold temperature reservoir for a heat engine. What is the maximum possible efficiency of an engine running between those two temperatures?
A) 4.7%
B) 5.1%
C) 7.9%
D) 15%
E) 30%
A) 4.7%
B) 5.1%
C) 7.9%
D) 15%
E) 30%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck


