Deck 20: Energy Transferred Thermally
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 20: Energy Transferred Thermally
1
A container of ideal gas has a movable frictionless piston.This container is placed in a very large water bath and slowly compressed so that the temperature of the gas remains constant and equal to the temperature of the water.Which of the following statements about this gas are true for this process? (There may be more than one correct choice.)
A) Heat leaves the gas during the compression.
B) Since the gas and water are at the same temperature, no heat can flow between them, which makes this an adiabatic compression.
C) The internal (thermal) energy of the gas does not change during the compression.
D) The internal energy of the gas increases during the compression because work is done on the gas.
E) Since the temperature of the gas remains constant, the pressure of the gas must also remain constant.
A) Heat leaves the gas during the compression.
B) Since the gas and water are at the same temperature, no heat can flow between them, which makes this an adiabatic compression.
C) The internal (thermal) energy of the gas does not change during the compression.
D) The internal energy of the gas increases during the compression because work is done on the gas.
E) Since the temperature of the gas remains constant, the pressure of the gas must also remain constant.
Heat leaves the gas during the compression.
2
When a fixed amount of ideal gas goes through an isobaric expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
its temperature must increase.
3
The process shown in the T-V diagram in the figure is an 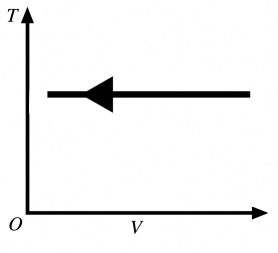
A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
E) isothermal expansion.

A) adiabatic compression.
B) isothermal compression.
C) isochoric compression.
D) isobaric compression.
E) isothermal expansion.
isothermal compression.
4
When a solid melts
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
When a fixed amount of ideal gas goes through an adiabatic expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature cannot change.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature cannot change.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
An ideal gas is compressed in a well-insulated chamber using a well-insulated piston.This process is
A) isochoric.
B) isothermal.
C) adiabatic.
D) isobaric.
A) isochoric.
B) isothermal.
C) adiabatic.
D) isobaric.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
An ideal gas increases in temperature from 22°C to 42°C by two different processes.In one process,the temperature increases at constant volume,and in the other process the temperature increases at constant pressure.Which of the following statements about this gas are correct? (There may be more than one correct choice.)
A) The heat required to cause this temperature change is the same for both the constant-volume and the constant-pressure processes.
B) More heat is required for the constant-pressure process than for the constant-volume process.
C) The change in the internal (thermal) energy of the gas is the same for both the constant-volume and the constant-pressure processes.
D) The root-mean-square (thermal) speed of the gas molecules increases more during the constant-volume process than during the constant-pressure process.
A) The heat required to cause this temperature change is the same for both the constant-volume and the constant-pressure processes.
B) More heat is required for the constant-pressure process than for the constant-volume process.
C) The change in the internal (thermal) energy of the gas is the same for both the constant-volume and the constant-pressure processes.
D) The root-mean-square (thermal) speed of the gas molecules increases more during the constant-volume process than during the constant-pressure process.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
(a)Internal human body temperature is often stated to be normal at 98.6°F. What is this temperature on the Celsius and Kelvin scales?
(b)Gallium boils at 2205°C.What is the corresponding temperature in the Fahrenheit and Kelvin scales?
(c)The boiling point of liquid nitrogen is 77.0 K.What is the corresponding temperature in the Fahrenheit and Celsius scales?
(b)Gallium boils at 2205°C.What is the corresponding temperature in the Fahrenheit and Kelvin scales?
(c)The boiling point of liquid nitrogen is 77.0 K.What is the corresponding temperature in the Fahrenheit and Celsius scales?

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
It is a well-known fact that water has a higher specific heat than iron.Now,consider equal masses of water and iron that are initially in thermal equilibrium.The same amount of heat,30 calories,is added to each one.Which statement is true?
A) They remain in thermal equilibrium.
B) They are no longer in thermal equilibrium; the iron is warmer.
C) They are no longer in thermal equilibrium; the water is warmer.
D) It is impossible to say without knowing the exact mass involved.
E) It is impossible to say without knowing the exact specific heats.
A) They remain in thermal equilibrium.
B) They are no longer in thermal equilibrium; the iron is warmer.
C) They are no longer in thermal equilibrium; the water is warmer.
D) It is impossible to say without knowing the exact mass involved.
E) It is impossible to say without knowing the exact specific heats.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
The process shown in the pV diagram in the figure is 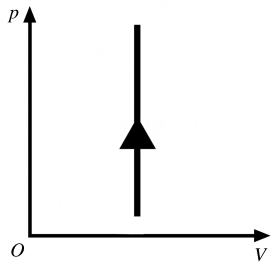
A) adiabatic.
B) isothermal.
C) isochoric.
D) isobaric.

A) adiabatic.
B) isothermal.
C) isochoric.
D) isobaric.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
An adiabatic compression is performed on an ideal gas.The final pressure is equal to 0.560 times the initial pressure and the final volume equals 1.50 times the initial volume.What is the adiabatic constant for the gas?
A) 1.33
B) 1.43
C) 1.48
D) 1.52
E) 1.67
A) 1.33
B) 1.43
C) 1.48
D) 1.52
E) 1.67

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
A quantity of ideal gas requires 800 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant volume. The same quantity of gas requires 900 kJ to raise the temperature of the gas by 10.0 K when the gas is maintained at constant pressure. What is the adiabatic constant γ for this gas?
A) 0.889
B) 1.13
C) 1.22
D) 1.67
E) 1.40
A) 0.889
B) 1.13
C) 1.22
D) 1.67
E) 1.40

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
When a gas undergoes an isothermal process,there is
A) no change in the pressure of the gas.
B) no change in the temperature of the gas.
C) no change in the volume of the gas.
D) no work done by (or on) the gas.
E) no heat added to the gas.
A) no change in the pressure of the gas.
B) no change in the temperature of the gas.
C) no change in the volume of the gas.
D) no work done by (or on) the gas.
E) no heat added to the gas.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
The process shown in the pV diagram in the figure is an 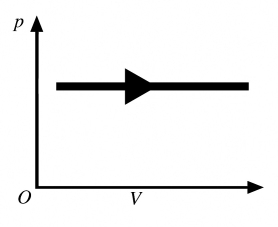
A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.

A) adiabatic expansion.
B) isothermal expansion.
C) isochoric expansion.
D) isobaric expansion.
E) isochoric compression.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
When a vapor condenses
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.
A) the temperature of the substance increases.
B) the temperature of the substance decreases.
C) heat energy leaves the substance.
D) heat energy enters the substance.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
When a fixed amount of ideal gas goes through an isochoric process
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must increase.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
Heat is added to a pure substance in a closed container at a constant rate.The figure shows a graph of the temperature of the substance as a function of time.If Lf = latent heat of fusion and Lv = latent heat of vaporization,what is the value of the ratio Lv / Lf for this substance? 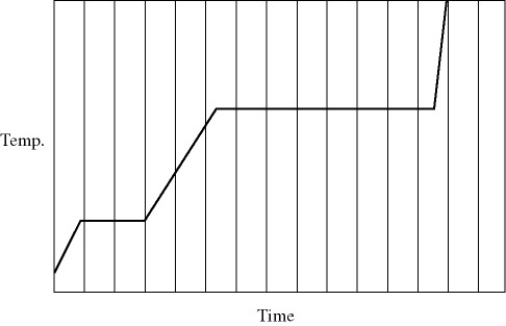
A) 5.0
B) 4.5
C) 7.2
D) 3.5
E) 1.5

A) 5.0
B) 4.5
C) 7.2
D) 3.5
E) 1.5

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper; the aluminum and the copper are in thermal contact.The specific heat of aluminum is more than double that of copper.Which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?
A) The copper experiences a greater temperature change.
B) The aluminum experiences a greater temperature change.
C) Neither; both objects experience the same magnitude temperature change.
D) It is impossible to tell without knowing the masses.
E) It is impossible to tell without knowing the volumes.
A) The copper experiences a greater temperature change.
B) The aluminum experiences a greater temperature change.
C) Neither; both objects experience the same magnitude temperature change.
D) It is impossible to tell without knowing the masses.
E) It is impossible to tell without knowing the volumes.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
When a fixed amount of ideal gas goes through an isothermal expansion
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must decrease.
E) its pressure must increase.
A) its internal (thermal) energy does not change.
B) the gas does no work.
C) no heat enters or leaves the gas.
D) its temperature must decrease.
E) its pressure must increase.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
Heat is added to a 2.0 kg piece of ice at a rate of 793.0 kW.How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
During an isothermal process,5.0 J of heat is removed from an ideal gas. How much work does the gas do during this process?
A) 0.00 J
B) 2.0 J
C) 5.0 J
D) -5.0 J
E) 10 J
A) 0.00 J
B) 2.0 J
C) 5.0 J
D) -5.0 J
E) 10 J

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
An ideal gas is allowed to expand slowly at constant temperature to twice its original volume. During the expansion,the gas absorbs 200 kJ of heat.
(a)What is the change in the internal (thermal)energy of the gas during the expansion?
(b)How much work does the gas do during the expansion?
(a)What is the change in the internal (thermal)energy of the gas during the expansion?
(b)How much work does the gas do during the expansion?

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
An ideal gas with γ = 1.67 is initially at 0°C in a volume of 10.0 L at a pressure of 1.00 atm.It is then expanded adiabatically to a volume of 10.4 L.What is the final temperature of the gas?
A) -7.1°C
B) 2.5°C
C) -23°C
D) 68°C
E) -20°C
A) -7.1°C
B) 2.5°C
C) -23°C
D) 68°C
E) -20°C

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
The gas in a perfectly insulated system does work at a rate of 13 W.At what rate is the internal (thermal)energy of the gas changing?
A) -13 W
B) 13 W
C) 0.00 W
D) 6.5 W
A) -13 W
B) 13 W
C) 0.00 W
D) 6.5 W

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
A system has a heat source supplying heat to an ideal gas at a rate of 187.0 W and the gas is doing work at a rate of 130.9 W.At what rate is the internal (thermal)energy of the gas changing?
A) 56.1 W
B) 318 W
C) -56.1 W
D) 187 W
A) 56.1 W
B) 318 W
C) -56.1 W
D) 187 W

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
During an isothermal process,5.0 J of heat is removed from an ideal gas. What is the change in internal (thermal)energy of the gas?
A) 0.00 J
B) 2.5 J
C) 5.0 J
D) 7.5 J
E) 10 J
A) 0.00 J
B) 2.5 J
C) 5.0 J
D) 7.5 J
E) 10 J

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
In an isochoric process,the internal (thermal)energy of an ideal gas decreases by 50 J. How much heat is exchanged with the gas during this process?
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
During an adiabatic process,an ideal gas does 25 J of work. What is the change in the internal (thermal)energy of the gas during this process?
A) 0.00 J
B) 50 J
C) 25 J
D) -25 J
E) -50 J
A) 0.00 J
B) 50 J
C) 25 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
In an isochoric process,the internal (thermal)energy of an ideal gas decreases by 50 J. How much work does the gas do during this process?
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J
A) 0.00 J
B) 25 J
C) 50 J
D) -25 J
E) -50 J

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
An ideal gas initially at 300 K and occupying a volume of 20 L is adiabatically compressed.If its final temperature is 400 K and γ = 1.30,what is its final volume?
A) 7.7 L
B) 14 L
C) 22 L
D) 52 L
A) 7.7 L
B) 14 L
C) 22 L
D) 52 L

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


