Deck 10: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/84
Play
Full screen (f)
Deck 10: Gases
1
Which two temperature changes are equivalent?
A)1 K = 1 F°
B)1 F° = 1 C°
C)1 C° = 1 K
D)none of the above
A)1 K = 1 F°
B)1 F° = 1 C°
C)1 C° = 1 K
D)none of the above
C
2
The root-mean-square speed of the molecules of an ideal gas is v.The gas is now slowly compressed to one-half its original volume with no change in temperature.What is the root-mean-square speed of the molecules now?
A)4v
B)2v
C)v/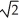
D)v
E)v/2
A)4v
B)2v
C)v/
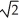
D)v
E)v/2
D
3
Two containers of equal volume each hold samples of the same ideal gas.Container A has twice as many molecules as container B.If the gas pressure is the same in the two containers,the correct statement regarding the absolute temperatures TA and TB in containers A and B,respectively,is
A)TA = TB.
B)TA = 2TB.
C)TA = TB.
TB.
D)TA =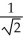 TB.
TB.
E)TA = TB.
TB.
A)TA = TB.
B)TA = 2TB.
C)TA =
 TB.
TB.D)TA =
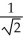 TB.
TB.E)TA =
 TB.
TB.C
4
When a heavy metal block is supported by a cylindrical vertical post of radius R,it exerts a force F on the post.If the diameter of the post is increased to 2R,what force does the block now exert on the post?
A)F/4
B)F/2
C)F/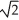
D)F
E)2F
A)F/4
B)F/2
C)F/
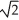
D)F
E)2F

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
5
A compressed gas with a total mass of 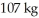 is stored in a spherical container having a radius of 0.521 m.What is the density of the compressed gas?
is stored in a spherical container having a radius of 0.521 m.What is the density of the compressed gas?
A)181 kg/m3
B)110 kg/m3
C)134 kg/m3
D)161 kg/m3
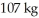 is stored in a spherical container having a radius of 0.521 m.What is the density of the compressed gas?
is stored in a spherical container having a radius of 0.521 m.What is the density of the compressed gas?A)181 kg/m3
B)110 kg/m3
C)134 kg/m3
D)161 kg/m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
6
When a box rests on a round sheet of wood on the ground,it exerts an average pressure p on the wood.If the wood is replaced by a sheet that has half the diameter of the original piece,what is the new average pressure?
A)4p
B)2p
C)p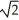
D)p/2
E)p/4
A)4p
B)2p
C)p
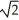
D)p/2
E)p/4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
7
Consider two equal-volume flasks of gas at the same temperature and pressure.One gas,oxygen,has a molecular mass of 32.The other gas,nitrogen,has a molecular mass of 28.What is the ratio of the number of oxygen molecules to the number of nitrogen molecules in these flasks?
A)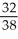
B)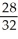
C)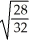
D)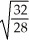
E)
A)
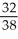
B)
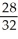
C)
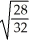
D)
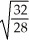
E)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
8
An ideal gas is held in a container of volume V at pressure p.The rms speed of a gas molecule under these conditions is v.If now the volume and pressure are changed to 2V and 2p,the rms speed of a molecule will be
A)v/2
B)v
C)2v
D)4v
E)v/4
A)v/2
B)v
C)2v
D)4v
E)v/4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
9
The absolute temperature of a gas is T.In order to double the rms speed of its molecules,what should be the new absolute temperature?
A)4T
B)2T
C)T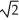
D)8T
E)16T
A)4T
B)2T
C)T
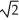
D)8T
E)16T

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
10
A sample of an ideal gas is heated and its Kelvin temperature doubles.If the root-mean-square speed of its molecules was originally v,what is the new root-mean-square speed?
A)4v
B)2v
C)v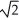
D)v/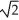
E)v/4
A)4v
B)2v
C)v
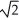
D)v/
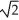
E)v/4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
11
Oxygen molecules are 16 times more massive than hydrogen molecules.At a given temperature,the average molecular kinetic energy of oxygen molecules,compared to that of hydrogen molecules,
A)is greater.
B)is less.
C)is the same.
D)cannot be determined without knowing the pressure and volume.
A)is greater.
B)is less.
C)is the same.
D)cannot be determined without knowing the pressure and volume.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
12
A styrofoam sphere of radius R has a density ρ.You now carefully compress the sphere so its radius is R/2.What is the density of the compressed sphere?
A)2 ρ
B)4 ρ
C)ρ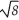
D)ρ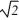
E)8 ρ
A)2 ρ
B)4 ρ
C)ρ
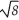
D)ρ
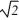
E)8 ρ

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
13
The temperature in your classroom is closest to
A)68 K.
B)68°C.
C)50°C.
D)295 K.
A)68 K.
B)68°C.
C)50°C.
D)295 K.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
14
A fixed container holds oxygen and helium gases at the same temperature.Which of the following statements are correct? (There could be more than one correct choice.)
A)The oxygen molecules have the greater average kinetic energy.
B)The helium molecules have the greater average kinetic energy.
C)The oxygen molecules have the greater speed.
D)The helium molecules have the greater speed.
E)The helium molecules have the same average kinetic as the oxygen molecules.
A)The oxygen molecules have the greater average kinetic energy.
B)The helium molecules have the greater average kinetic energy.
C)The oxygen molecules have the greater speed.
D)The helium molecules have the greater speed.
E)The helium molecules have the same average kinetic as the oxygen molecules.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
15
A mole of diatomic oxygen molecules and a mole of diatomic nitrogen molecules are at STP.Which statements are true about these molecules? (There could be more than one correct choice.)
A)Both gases have the same average molecular speeds.
B)Both gases have the same number of molecules.
C)Both gases have the same average kinetic energy per molecule.
D)Both gases have the same average momentum per molecule.
A)Both gases have the same average molecular speeds.
B)Both gases have the same number of molecules.
C)Both gases have the same average kinetic energy per molecule.
D)Both gases have the same average momentum per molecule.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
16
A plastic block of dimensions 2.00 cm × 3.00 cm × 4.00 cm has a mass of 30.0 g.What is its density?
A)0.80 g/cm3
B)1.20 g/cm3
C)1.25 g/cm3
D)1.60 g/cm3
A)0.80 g/cm3
B)1.20 g/cm3
C)1.25 g/cm3
D)1.60 g/cm3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
17
The absolute temperature of an ideal gas is directly proportional to which of the following quantities?
A)the average speed of its molecules
B)the average momentum of its molecules
C)the average kinetic energy of its molecules
D)the mass of its molecules
E)It is proportional to all of the above quantities.
A)the average speed of its molecules
B)the average momentum of its molecules
C)the average kinetic energy of its molecules
D)the mass of its molecules
E)It is proportional to all of the above quantities.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
18
A hollow sphere of negligible mass and radius R is completely filled with a liquid so that its density is ρ.You now enlarge the sphere so its radius is 2R and completely fill it with the same liquid.What is the density of the enlarged sphere?
A)8 ρ
B)4 ρ
C)ρ/2
D)ρ
E)ρ/8
A)8 ρ
B)4 ρ
C)ρ/2
D)ρ
E)ρ/8

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
19
Oxygen molecules are 16 times more massive than hydrogen molecules.At a given temperature,how do their average molecular speeds compare? The oxygen molecules are moving
A)four times faster than the hydrogen molecules.
B)at 1/4 the speed of the hydrogen molecules.
C)sixteen times faster than the hydrogen molecules.
D)at 1/16 the speed of the hydrogen molecules.
E)at 1/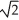 the speed of the hydrogen molecules.
the speed of the hydrogen molecules.
A)four times faster than the hydrogen molecules.
B)at 1/4 the speed of the hydrogen molecules.
C)sixteen times faster than the hydrogen molecules.
D)at 1/16 the speed of the hydrogen molecules.
E)at 1/
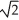 the speed of the hydrogen molecules.
the speed of the hydrogen molecules.
Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
20
Substance A has a density of 3 g/cm3 and substance B has a density of 4 g/cm3.In order to obtain equal masses of these two substances,what must be the ratio of the volume of A to the volume of B?
A)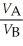 =
= 
B)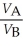 =
= 
C)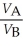 =
= 
D)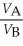 =
= 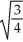
E)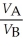 =
= 
A)
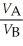 =
= 
B)
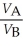 =
= 
C)
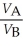 =
= 
D)
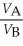 =
= 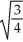
E)
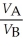 =
= 

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
21
Express a body temperature 98.6°F in Celsius degrees.
A)37.0°C
B)45.5°C
C)66.6°C
D)72.6°C
A)37.0°C
B)45.5°C
C)66.6°C
D)72.6°C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the pressure exerted on the ground due to 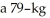 person standing on one foot if the bottom of the person's foot is
person standing on one foot if the bottom of the person's foot is 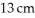 wide and
wide and  long.
long.
A)2.1 × 104 Pa
B)2.2 × 103 Pa
C)4.8 × 104 Pa
D)5.3 × 104 Pa
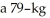 person standing on one foot if the bottom of the person's foot is
person standing on one foot if the bottom of the person's foot is 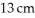 wide and
wide and  long.
long.A)2.1 × 104 Pa
B)2.2 × 103 Pa
C)4.8 × 104 Pa
D)5.3 × 104 Pa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
23
One of the dangers of tornados and hurricanes is the rapid drop in air pressure that is associated with such storms.Assume that the air pressure inside of a sealed house is 1.02 atm when a hurricane hits.The hurricane rapidly decreases the external air pressure to 0.910 atm.What net outward force is exerted on a square window of the house that is 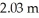 on each side? (1.00 atm = 1.01 × 105 Pa)
on each side? (1.00 atm = 1.01 × 105 Pa)
A)4.59 × 104 N
B)5.19 × 104 N
C)4.82 × 105 N
D)5.42 × 105 N
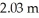 on each side? (1.00 atm = 1.01 × 105 Pa)
on each side? (1.00 atm = 1.01 × 105 Pa)A)4.59 × 104 N
B)5.19 × 104 N
C)4.82 × 105 N
D)5.42 × 105 N

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
24
The density of material at the center of a neutron star is approximately 1.00 × 1018 kg/m3.What is the mass of a cube of this material that is 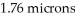 on each side.(One micron is equal to 1.00 × 10-6 m.)
on each side.(One micron is equal to 1.00 × 10-6 m.)
A)5.45 kg
B)4.74 kg
C)6.16 kg
D)6.70 kg
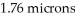 on each side.(One micron is equal to 1.00 × 10-6 m.)
on each side.(One micron is equal to 1.00 × 10-6 m.)A)5.45 kg
B)4.74 kg
C)6.16 kg
D)6.70 kg

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
25
Oxygen condenses into a liquid at approximately 90 K.What temperature,in degrees Fahrenheit,does this correspond to?
A)-193°F
B)-217°F
C)-265°F
D)-297°F
A)-193°F
B)-217°F
C)-265°F
D)-297°F

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
26
A 50.0-N brick has the following measures: 30.0 cm × 10.0 cm × 4.00 cm.What is the maximum pressure it can exert on a horizontal surface due to its interaction with Earth?
A)1.25 Pa
B)12.5 Pa
C)1.25 kPa
D)12.5 kPa
A)1.25 Pa
B)12.5 Pa
C)1.25 kPa
D)12.5 kPa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
27
Express -40°C in °F.
A)-72°F
B)-54°F
C)-40°F
D)4.4°F
A)-72°F
B)-54°F
C)-40°F
D)4.4°F

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
28
A 900-N person is standing on snowshoes.Each snowshoe has area 2500 cm2 and has negligible weight.What pressure does this person's exert on the snow due to his interaction with Earth?
A)0.18 N/m2
B)0.36 N/m2
C)1800 N/m2
D)3600 N/m2
A)0.18 N/m2
B)0.36 N/m2
C)1800 N/m2
D)3600 N/m2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
29
The temperature changes from 35°F during the night to 75°F during the day.What is the temperature change on the Celsius scale?
A)72 C°
B)40 C°
C)32 C°
D)22 C°
A)72 C°
B)40 C°
C)32 C°
D)22 C°

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
30
A 1200-kg car is supported equally by the four tires,which are inflated to the same gauge pressure.What gauge pressure is required so the area of contact of each tire with the road is 100 cm2?
A)12 × 105 Pa
B)12 × 104 Pa
C)2.9 × 105 Pa
D)2.9 × 104 Pa
A)12 × 105 Pa
B)12 × 104 Pa
C)2.9 × 105 Pa
D)2.9 × 104 Pa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
31
Nitrogen boils at -196°C.What is the corresponding temperature in the Fahrenheit scale?
A)-315°F
B)-196°F
C)-346°F
D)-290°F
E)-321°F
A)-315°F
B)-196°F
C)-346°F
D)-290°F
E)-321°F

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
32
The weather outside is frightful.The temperature is -22°F.What is the corresponding temperature in the Celsius scale?
A)-35°C
B)-30°C
C)-22°C
D)-20°C
E)-12°C
A)-35°C
B)-30°C
C)-22°C
D)-20°C
E)-12°C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
33
At what,if any,temperature are the numerical readings on the Fahrenheit and Celsius scales the same?
A)-30°
B)-40°
C)-50°
D)-60°
E)They can never read the same because they are based on different zeroes.
A)-30°
B)-40°
C)-50°
D)-60°
E)They can never read the same because they are based on different zeroes.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
34
A temperature change of 20 C° corresponds to a Fahrenheit temperature change of
A)68 F°.
B)11 F°.
C)36 F°.
D)18 F°.
A)68 F°.
B)11 F°.
C)36 F°.
D)18 F°.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
35
A 100-kg person sits on a 5-kg bicycle.The force that the person exerts on the bicycle is borne equally by the two wheels of the bicycle.The tires are 2.0 cm wide and are inflated to a gauge pressure of 8.0 × 105 Pa.What length of each tire is in contact with the ground?
A)1.6 cm
B)1.8 cm
C)3.2 cm
D)2.4 cm
E)6.4 cm
A)1.6 cm
B)1.8 cm
C)3.2 cm
D)2.4 cm
E)6.4 cm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
36
How many grams of ethanol (density 0.80 g/cm3)should be added to 5.0 g of chloroform (density 1.5 g/cm3)if the resulting mixture is to have a density of 1.2 g/cm3? Assume that the fluids do not change their volumes when they are mixed.
A)2.0 g
B)2.4 g
C)1.8 g
D)4.4 g
E)1.6 g
A)2.0 g
B)2.4 g
C)1.8 g
D)4.4 g
E)1.6 g

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
37
A 1938 nickel has a diameter of 21.21 mm,a thickness of 1.95 mm,and weighs 0.04905 N.What is its density?
A)1.37 × 10-4 kg/m3
B)1.82 × 103 kg/m3
C)19.3 × 103 kg/m3
D)3.63 × 103 kg/m3
E)7.26 × 103 kg/m3
A)1.37 × 10-4 kg/m3
B)1.82 × 103 kg/m3
C)19.3 × 103 kg/m3
D)3.63 × 103 kg/m3
E)7.26 × 103 kg/m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
38
What is absolute zero on the (a)Celsius scale and (b)on the Fahrenheit scale?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
39
Under standard conditions,the density of air is 1.29 kg/m3.What is the mass of the air inside a room measuring 4.0 m × 3.0 m × 2.0 m?
A)0.32 kg
B)3.1 kg
C)31 kg
D)1.9 kg
E)19 kg
A)0.32 kg
B)3.1 kg
C)31 kg
D)1.9 kg
E)19 kg

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
40
A round coin has a diameter of 19.55 mm,a thickness of 1.55 mm,and a mass of 2.50 g.What is its density?
A)5.37 × 103 kg/m3
B)21.5 × 103 kg/m3
C)1.34 × 103 kg/m3
D)2.68 × 103 kg/m3
E)1.86 × 10-4 kg/m3
A)5.37 × 103 kg/m3
B)21.5 × 103 kg/m3
C)1.34 × 103 kg/m3
D)2.68 × 103 kg/m3
E)1.86 × 10-4 kg/m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
41
A gas-filled vertical cylinder,closed at the bottom end,is fitted at the top with a piston that can move freely.The mass of the piston is 10.0 kg,and the initial height of the piston above the bottom of the cylinder is 25 cm.A mass of 8.0 kg is placed on the piston.What is the resulting height of the piston,assuming that the temperature of the ideal gas is kept constant?
A)12 cm
B)13 cm
C)14 cm
D)15 cm
E)16 cm
A)12 cm
B)13 cm
C)14 cm
D)15 cm
E)16 cm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
42
A refrigerator has an interior volume of 0.500 m3.The temperature inside the refrigerator in 282 K,and the pressure is 101 kPa.If the molecular weight of air is 29 g/mol,what is the mass of air inside the refrigerator? (R = 8.31 J/mol × K)
A)625 g
B)513 g
C)447 g
D)329 g
E)243 g
A)625 g
B)513 g
C)447 g
D)329 g
E)243 g

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
43
A car starts out when the air temperature is 288 K and the absolute (total)air pressure in the tires is 500 kPa.After driving a while,the temperature of the air in the tires increases to 298 K.What is the pressure in the tires at that point,assuming their volume does not change?
A)129 kPa
B)483 kPa
C)507 kPa
D)517 kPa
E)532 kPa
A)129 kPa
B)483 kPa
C)507 kPa
D)517 kPa
E)532 kPa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
44
A weather balloon containing 2.0 m3 of hydrogen gas rises from a location at which the temperature is 22°C and the pressure is 101 kPa to a location where the temperature is -39°C and the pressure is 20 kPa.If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure,what is the new volume of the balloon?
A)4.0 m3
B)6.0 m3
C)8.0 m3
D)10 m3
E)12 m3
A)4.0 m3
B)6.0 m3
C)8.0 m3
D)10 m3
E)12 m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
45
How many molecules are in (a)1.0 cm3 of air at STP and (b)1.0 cm3 of helium at STP? (R = 8.31 J/mol ∙ K,NA = 6.022 × 1023 molecules/mol)

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
46
A vertical cylinder,closed at the bottom end,contains 0.0100 mol of ideal gas.It is fitted at the top with a piston that can move freely.The mass of the piston is 14.0 kg and the initial height of the piston above the bottom of the cylinder is 25 cm.What is the temperature of the gas? (R = 8.31 J/mol ∙ K)
A)290 K
B)413 K
C)3620 K
D)405 K
E)500 K
A)290 K
B)413 K
C)3620 K
D)405 K
E)500 K

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
47
Your lungs hold 4.2 L of air at a temperature of 27°C and a pressure of 101.3 kPa.How many moles of air do your lungs hold? (R = 8.31 J/mol ∙ K)
A)0.15 moles
B)0.17 moles
C)0.19 moles
D)0.21 moles
E)0.23 moles
A)0.15 moles
B)0.17 moles
C)0.19 moles
D)0.21 moles
E)0.23 moles

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
48
A balloon originally has a volume of 1.0 m3 when the gas in it is at 20°C and under a pressure of 1.0 atm.As it rises in the earth's atmosphere,its volume expands.What will be its new volume if its final temperature and pressure are -40°C and 0.10 atm?
A)2.0 m3
B)4.0 m3
C)6.0 m3
D)8.0 m3
A)2.0 m3
B)4.0 m3
C)6.0 m3
D)8.0 m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
49
Platinum melts at 3215°F.What is the corresponding temperature in the Kelvin scale?
A)2041 K
B)2135 K
C)2207 K
D)2296 K
E)3215 K
A)2041 K
B)2135 K
C)2207 K
D)2296 K
E)3215 K

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
50
If a certain sample of an ideal gas has a temperature of 104°C and exerts a pressure of 2.3 × 104 Pa on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is R = 8.31 J/mol × K and Avogadro's number is NA = 6.022 × 1023 molecules/mol.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
51
A certain automobile tire has a volume of 0.0185 m3.If the absolute (or total)pressure in the tire is 500 kPa and the temperature is 298 K,how many molecules are there inside the tire? (R = 8.31 J/mol ∙ K,NA = 6.022 x 1023 molecules/mol)
A)2.25 × 1023 molecules
B)2.25 × 1024 molecules
C)3.25 × 1023 molecules
D)3.25 × 1024 molecules
E)3.25 × 1025 molecules
A)2.25 × 1023 molecules
B)2.25 × 1024 molecules
C)3.25 × 1023 molecules
D)3.25 × 1024 molecules
E)3.25 × 1025 molecules

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
52
A quantity of an ideal gas is kept in a rigid container of constant volume.If the gas is originally at a temperature of 19°C,at what temperature will the pressure of the gas double from its original value?
A)91°C
B)38°C
C)311°C
D)273°C
E)122°C
A)91°C
B)38°C
C)311°C
D)273°C
E)122°C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
53
A laboratory vacuum pump can reduce the pressure in a chamber to 1.0 × 10-7 Pa.If the volume of the chamber is 0.500 m3 and the temperature is 27°C,how many molecules are left inside the chamber? (NA = 6.022 × 1023 molecules/mol,R = 8.31 J/mol ∙ K)
A)1.2 × 1013
B)2.4 × 1013
C)1.2 × 1012
D)2.4 × 1012
E)1.2 × 1014
A)1.2 × 1013
B)2.4 × 1013
C)1.2 × 1012
D)2.4 × 1012
E)1.2 × 1014

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
54
On a cold day,you take in 4.2 L of air into your lungs at a temperature of 0°C.If you hold your breath until the temperature of the air in your lungs reaches 37°C,what is the volume of the air in your lungs at that point,assuming the pressure does not change?
A)4.2 L
B)4.4 L
C)4.6 L
D)4.8 L
E)5.0 L
A)4.2 L
B)4.4 L
C)4.6 L
D)4.8 L
E)5.0 L

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
55
A 20.0-L pressure vessel holds 2.00 mol of oxygen at 30°C.What is the pressure inside the vessel? (R = 8.31 J/mol ∙ K)
A)101 Pa
B)101 kPa
C)1.01 MPa
D)2.52 MPa
E)252 kPa
A)101 Pa
B)101 kPa
C)1.01 MPa
D)2.52 MPa
E)252 kPa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
56
A jar holds 2.0 L of ideal nitrogen gas,N2,at STP.The atomic mass of nitrogen is 14.0 g/mol,the ideal gas constant is R = 8.31 J/mol ∙ K,Avogadro's number is NA = 6.022 × 1023 molecules/mol,and 1.00 atm = 101 kPa.
(a)How many moles of nitrogen are in the jar?
(b)How many nitrogen molecules are in the jar?
(c)What is the mass of the nitrogen in the jar?
(a)How many moles of nitrogen are in the jar?
(b)How many nitrogen molecules are in the jar?
(c)What is the mass of the nitrogen in the jar?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles are there in 2.00 kg of copper? The atomic mass of copper is 63.5 g/mol and its density is 8.90 g/cm3.
A)15.3
B)31.5
C)51.3
D)53.1
A)15.3
B)31.5
C)51.3
D)53.1

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
58
An ideal gas occupies 6.00 × 102 cm3 at 20°C.At what temperature will it occupy 1.20 × 103 cm3 if the pressure is held constant?
A)10°C
B)40°C
C)100°C
D)313°C
A)10°C
B)40°C
C)100°C
D)313°C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
59
Originally 2.00 mol of gas are at standard temperature and pressure If the temperature changes to 47.0°C and the pressure decreases to half of what it was,how many liters do the two moles now occupy? (1 atm = 101 kPa,R = 8.31 J/mol ∙ K)

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
60
An ideal gas has a pressure of 2.5 atm,a volume of 1.0 L at a temperature of 30°C.How many molecules are there in this gas? (R = 8.31 J/mol ∙ K,1.00 atm = 101 kPa,NA = 6.022 × 1023)
A)6.1 × 1023
B)6.0 × 1022
C)2.4 × 1022
D)2.3 × 1023
A)6.1 × 1023
B)6.0 × 1022
C)2.4 × 1022
D)2.3 × 1023

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
61
What is the total translational kinetic energy of the gas in a classroom filled with nitrogen at 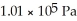 at
at 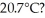 The dimensions of the classroom are
The dimensions of the classroom are 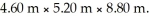 The Boltzmann constant is 1.3806503 × 10-23 J/K,R = 8.31 J/mol ∙ K,and NA = 6.022 × 1023 molecules/mol.
The Boltzmann constant is 1.3806503 × 10-23 J/K,R = 8.31 J/mol ∙ K,and NA = 6.022 × 1023 molecules/mol.
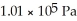 at
at 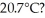 The dimensions of the classroom are
The dimensions of the classroom are 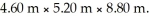 The Boltzmann constant is 1.3806503 × 10-23 J/K,R = 8.31 J/mol ∙ K,and NA = 6.022 × 1023 molecules/mol.
The Boltzmann constant is 1.3806503 × 10-23 J/K,R = 8.31 J/mol ∙ K,and NA = 6.022 × 1023 molecules/mol.
Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
62
A sealed container holds 0.020 moles of ideal nitrogen (N2)gas,at a pressure of 1.5 atm and a temperature of 290 K.The atomic mass of nitrogen is 14.0 g/mol.What is the average translational kinetic energy of a nitrogen molecule? The Boltzmann constant is 1.38 × 10-23 J/K.
A)4.0 ×1021 J
B)6.0 ×1021 J
C)8.0 ×1021 J
D)10 ×1021 J
E)12 ×1021 J
A)4.0 ×1021 J
B)6.0 ×1021 J
C)8.0 ×1021 J
D)10 ×1021 J
E)12 ×1021 J

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
63
The molecular weight of nitrogen,N2,is 28 g/mol.What is the rms speed of nitrogen molecules in a cooler at 8.0°C? The Boltzmann constant is 1.38 × 10-23 J/K and NA = 6.022 × 1023 molecules/mol.
A)450 m/s
B)500 m/s
C)550 m/s
D)600 m/s
E)650 m/s
A)450 m/s
B)500 m/s
C)550 m/s
D)600 m/s
E)650 m/s

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
64
A 24.0-L tank contains ideal helium gas at 27°C and a pressure of 22.0 atm.How many moles of gas are in the tank? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)
A)238 mol
B)138 mol
C)17.5 mol
D)21.4 mol
E)76.0 mol
A)238 mol
B)138 mol
C)17.5 mol
D)21.4 mol
E)76.0 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
65
What is the average translational kinetic energy of an ideal gas at 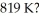 The Boltzmann constant is 1.38 × 10-23 J/K.
The Boltzmann constant is 1.38 × 10-23 J/K.
A)1.70 x 10-20 J
B)5.65 x 10-21 J
C)1.13 x 10-17 J
D)3.77 x 10-19 J
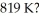 The Boltzmann constant is 1.38 × 10-23 J/K.
The Boltzmann constant is 1.38 × 10-23 J/K.A)1.70 x 10-20 J
B)5.65 x 10-21 J
C)1.13 x 10-17 J
D)3.77 x 10-19 J

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
66
At what temperature would the root mean square speed of oxygen molecules,O2,be 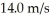 if oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
if oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
A)0.251 K
B)2090 K
C)6270 K
D)1.52 × 1023 K
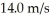 if oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
if oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.A)0.251 K
B)2090 K
C)6270 K
D)1.52 × 1023 K

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
67
If the temperature of a gas is increased from 20°C to 100°C,by what factor does the rms speed of an ideal molecule change?
A)1.1
B)1.3
C)2.2
D)1.6
A)1.1
B)1.3
C)2.2
D)1.6

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
68
A flask contains a mixture of argon and neon gases at a stabilized temperature.The root-mean-square speed of the argon gas is determined to be 1.21 km/s.What is the root-mean-square speed of the neon gas? The atomic mass of argon is 39.95 g/mol,and that of neon is 20.18 g/mol.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
69
A 5.3 L flask of ideal neon gas (which is monatomic)is at a pressure of 6.0 atm and a temperature of 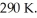 The atomic mass of neon is 20.2 g/mol.What is the mass of the neon gas in the flask.(R = 8.31 J/mol ∙ K,1 atm = 101 kPa,NA = 6.022 × 1023 molecules/mol)
The atomic mass of neon is 20.2 g/mol.What is the mass of the neon gas in the flask.(R = 8.31 J/mol ∙ K,1 atm = 101 kPa,NA = 6.022 × 1023 molecules/mol)
A)2.7 × 10-2
B)1.6 × 10-2
C)1.3 × 101
D)2.7 × 101
E)2.7 × 103
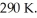 The atomic mass of neon is 20.2 g/mol.What is the mass of the neon gas in the flask.(R = 8.31 J/mol ∙ K,1 atm = 101 kPa,NA = 6.022 × 1023 molecules/mol)
The atomic mass of neon is 20.2 g/mol.What is the mass of the neon gas in the flask.(R = 8.31 J/mol ∙ K,1 atm = 101 kPa,NA = 6.022 × 1023 molecules/mol)A)2.7 × 10-2
B)1.6 × 10-2
C)1.3 × 101
D)2.7 × 101
E)2.7 × 103

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
70
A sealed container holds 0.020 moles of ideal nitrogen (N2)gas,at a pressure of 1.5 atm and a temperature of 290 K.The atomic mass of nitrogen is 14.0 g/mol.How many molecules of nitrogen are in the container? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)
A)1.5 × 1021 mol
B)3.0 × 1021 mol
C)6.0 × 1021 mol
D)1.2 × 1022 mol
E)2.4 × 1022 mol
A)1.5 × 1021 mol
B)3.0 × 1021 mol
C)6.0 × 1021 mol
D)1.2 × 1022 mol
E)2.4 × 1022 mol

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
71
A 0.50 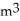 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of
gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 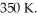 The atomic mass of nitrogen is
The atomic mass of nitrogen is 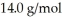 What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K,NA = 6.022 × 1023 molecules/mol.)
What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K,NA = 6.022 × 1023 molecules/mol.)
A)560
B)790
C)390
D)21
E)97
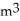 gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of
gas tank holds 3.0 moles of ideal diatomic nitrogen gas at a temperature of 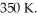 The atomic mass of nitrogen is
The atomic mass of nitrogen is 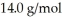 What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K,NA = 6.022 × 1023 molecules/mol.)
What is the rms speed of the molecules? (The Boltzmann constant is 1.38 × 10-23 J/K,NA = 6.022 × 1023 molecules/mol.)A)560
B)790
C)390
D)21
E)97

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
72
At what temperature is the rms speed of hydrogen molecules,H2,which have a molecular weight of 2.02 g/mole,equal to 2.0 km/s? The Boltzmann constant is 1.38 × 10-23 J/K and NA = 6.022 × 1023 molecules/mol.
A)17°C
B)34°C
C)51°C
D)68°C
E)72°C
A)17°C
B)34°C
C)51°C
D)68°C
E)72°C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
73
A 4.2-L flask of ideal neon gas (which is monatomic)is at a pressure of 3.3 atm and a temperature of 450 K.The atomic mass of neon is 20.2 g/mol.How many neon atoms are in the flask? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa,NA = 6.022 × 1023 molecules/mol)
A)2.3 × 1023
B)2.3 × 1022
C)6.9 × 1023
D)2.3 × 1025
E)6.9 × 1022
A)2.3 × 1023
B)2.3 × 1022
C)6.9 × 1023
D)2.3 × 1025
E)6.9 × 1022

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
74
An oxygen molecule,O2,falls in a vacuum.From what height must it fall so that its translational kinetic energy at the bottom of its fall equals the average translational kinetic energy of an oxygen molecule at 920 K? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.Neglect air resistance and assume that g remains constant at 9.8 m/s2 throughout the fall of the molecule.
A)49 km
B)12 km
C)24 km
D)37 km
E)5.2 km
A)49 km
B)12 km
C)24 km
D)37 km
E)5.2 km

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
75
The rms speed of a certain sample of carbon dioxide molecules,with a molecular weight of 44.0 g/mole,is 396 m/s.What is the rms speed of water vapor molecules,with a molecular weight of 18.0 g/mol,at the same temperature as the carbon dioxide?
A)253 m/s
B)387 m/s
C)421 m/s
D)506 m/s
E)619 m/s
A)253 m/s
B)387 m/s
C)421 m/s
D)506 m/s
E)619 m/s

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
76
What is the average translational kinetic energy of a nitrogen molecule in the air in a room in which the air temperature is 17°C? The Boltzmann constant is 1.38 × 10-23 J/K.
A)6.01 × 10-21 J
B)4.00 × 10-21 J
C)5.00 × 10-21 J
D)7.00 × 10-21 J
E)9.00 × 10-21 J
A)6.01 × 10-21 J
B)4.00 × 10-21 J
C)5.00 × 10-21 J
D)7.00 × 10-21 J
E)9.00 × 10-21 J

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
77
A sealed cylinder fitted with a movable piston contains ideal gas at 27°C,pressure 0.500 × 105 Pa,and volume 1.25 m3.What will be the final temperature if the gas is compressed to 0.800 m3 and the pressure rises to 0.820 × 105 Pa?
A)42°C
B)68°C
C)130°C
D)250°C
E)150°C
A)42°C
B)68°C
C)130°C
D)250°C
E)150°C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
78
A 3.9-L volume of ideal neon gas (monatomic)is at a pressure of 5.6 aym and a temperature of 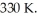 The atomic mass of neon is
The atomic mass of neon is 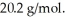 The temperature of the gas is now increased to 430 K and the volume is increased to
The temperature of the gas is now increased to 430 K and the volume is increased to 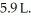 What is the final pressure of the gas?
What is the final pressure of the gas?
A)4.8 atm
B)4.3 atm
C)5.3 atm
D)5.8 atm
E)6.3 atm
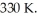 The atomic mass of neon is
The atomic mass of neon is 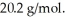 The temperature of the gas is now increased to 430 K and the volume is increased to
The temperature of the gas is now increased to 430 K and the volume is increased to 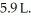 What is the final pressure of the gas?
What is the final pressure of the gas?A)4.8 atm
B)4.3 atm
C)5.3 atm
D)5.8 atm
E)6.3 atm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
79
(a)At what Celsius temperature is the average kinetic energy of a helium gas atom equal to 6.21 × 10-21 J? The Boltzmann constant is 1.38 × 10-23 J/K .
(b)What would be the temperature for radon gas?
(b)What would be the temperature for radon gas?

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
80
A 0.40- 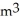 gas tank holds 7.0 moles of ideal diatomic nitrogen gas at a temperature of
gas tank holds 7.0 moles of ideal diatomic nitrogen gas at a temperature of 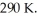 The atomic mass of nitrogen is
The atomic mass of nitrogen is 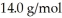 What is the pressure of the gas? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)
What is the pressure of the gas? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)
A)42 atm
B)37 atm
C)32 atm
D)27 atm
E)22 atm
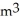 gas tank holds 7.0 moles of ideal diatomic nitrogen gas at a temperature of
gas tank holds 7.0 moles of ideal diatomic nitrogen gas at a temperature of 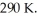 The atomic mass of nitrogen is
The atomic mass of nitrogen is 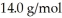 What is the pressure of the gas? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)
What is the pressure of the gas? (R = 8.31 J/mol ∙ K,1 atm = 101 kPa)A)42 atm
B)37 atm
C)32 atm
D)27 atm
E)22 atm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck


