Deck 14: Second Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 14: Second Law of Thermodynamics
1
A thermodynamic engine absorbs 64 kcal of energy each cycle and exhausts 42 kcal.
(a)What is the efficiency of this engine?
(b)How much work does this engine do per cycle?
(a)What is the efficiency of this engine?
(b)How much work does this engine do per cycle?
(a)34%
(b)22 kcal
(b)22 kcal
2
A thermodynamic engine receives 7000 J of energy and loses 3000 J in each cycle.What is the efficiency of this engine?
A)57%
B)30%
C)70%
D)43%
A)57%
B)30%
C)70%
D)43%
A
3
The figure shows a pV diagram for a cycle of a thermodynamic engine for which QH = 59 J.What is the thermal efficiency of the engine? 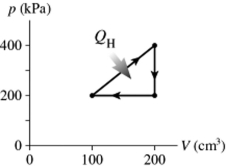
A)17%
B)34%
C)8.5%
D)14%

A)17%
B)34%
C)8.5%
D)14%
A
4
If the efficiency of a Carnot engine were to be 100%,the cool reservoirs would have to be
A)at absolute zero.
B)at 0°C.
C)at 100°C.
D)infinitely hot.
A)at absolute zero.
B)at 0°C.
C)at 100°C.
D)infinitely hot.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
An ideal Carnot thermodynamic engine operates between reservoirs at 1740 K and 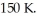 In each cycle,260 J of thermal energy is rejected to the low temperature reservoir.In each cycle,how much mechanical work W is performed by the engine?
In each cycle,260 J of thermal energy is rejected to the low temperature reservoir.In each cycle,how much mechanical work W is performed by the engine?
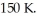 In each cycle,260 J of thermal energy is rejected to the low temperature reservoir.In each cycle,how much mechanical work W is performed by the engine?
In each cycle,260 J of thermal energy is rejected to the low temperature reservoir.In each cycle,how much mechanical work W is performed by the engine?
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
An ideal gas undergoes an isothermal expansion.During this process,its entropy
A)decreases.
B)remains unchanged.
C)increases.
D)cannot be predicted from the data given.
A)decreases.
B)remains unchanged.
C)increases.
D)cannot be predicted from the data given.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
An important feature of the Carnot cycle is that
A)its efficiency can be 100%.
B)its efficiency depends only on the absolute temperature of the hot reservoir used.
C)its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used, and on nothing else.
D)it is an example of an irreversible process that can be analyzed exactly without approximations.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.
A)its efficiency can be 100%.
B)its efficiency depends only on the absolute temperature of the hot reservoir used.
C)its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used, and on nothing else.
D)it is an example of an irreversible process that can be analyzed exactly without approximations.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
A thermodynamic engine has an efficiency of 35.0% and receives 150 J of energy per cycle.
(a)How much work does it do in each cycle?
(b)How much thermal energy does it "waste" in each cycle?
(a)How much work does it do in each cycle?
(b)How much thermal energy does it "waste" in each cycle?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
A certain thermodynamic engine extracts 1.30 kJ of thermal energy from a hot temperature reservoir and discharges 0.70 kJ of energy to a cold temperature reservoir.What is the efficiency of this engine?
A)46%
B)54%
C)86%
D)27%
E)13%
A)46%
B)54%
C)86%
D)27%
E)13%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
A nuclear power plant has an actual efficiency of 33%.If 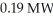 of energy are released from fission,how much electric power does the power plant produce?
of energy are released from fission,how much electric power does the power plant produce?
A)0.063 MW
B)6.3 MW
C)25 MW
D)0.25 MW
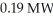 of energy are released from fission,how much electric power does the power plant produce?
of energy are released from fission,how much electric power does the power plant produce?A)0.063 MW
B)6.3 MW
C)25 MW
D)0.25 MW

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
A gas follows the pV trajectory shown in Figure 16.2.How much work is done per cycle by the gas if the gas in a thermodynamic engine follows the cycle shown in the pV diagram.How much work does this engine do each cycle if p0 = 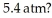
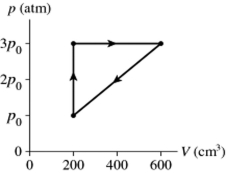
A)220 J
B)440 J
C)870 J
D)1100 J
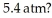

A)220 J
B)440 J
C)870 J
D)1100 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
A certain automobile engine takes in 4.00 kJ of energy and performs 1.10 kJ of mechanical work in each cycle.
(a)Calculate the engine's efficiency.
(b)How much thermal energy is "wasted" in each cycle?
(a)Calculate the engine's efficiency.
(b)How much thermal energy is "wasted" in each cycle?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a false statement?
A)Entropy is a quantitative measure of disorder.
B)The total entropy change in one cycle of a Carnot engine is zero.
C)The entropy of an isolated system remains constant.
D)Entropy can be measured in units of J/K.
A)Entropy is a quantitative measure of disorder.
B)The total entropy change in one cycle of a Carnot engine is zero.
C)The entropy of an isolated system remains constant.
D)Entropy can be measured in units of J/K.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following is a true statement?
A)The second law of thermodynamics is a consequence of the first law of thermodynamics.
B)It is possible for thermal energy to flow spontaneously from a hot body to a cold one or from a cold one to a hot one, depending on whether or not the process is reversible or irreversible.
C)It is not possible to convert work entirely into thermal energy.
D)It is impossible to transfer thermal energy from a cooler to a hotter body.
E)All of these statements are false.
A)The second law of thermodynamics is a consequence of the first law of thermodynamics.
B)It is possible for thermal energy to flow spontaneously from a hot body to a cold one or from a cold one to a hot one, depending on whether or not the process is reversible or irreversible.
C)It is not possible to convert work entirely into thermal energy.
D)It is impossible to transfer thermal energy from a cooler to a hotter body.
E)All of these statements are false.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
An ideal Carnot engine is operated between a hot and a cold reservoir.The temperature difference between the two reservoirs is 284°C.If the efficiency of this ideal engine is 24.0%,find the temperature of the cold reservoir in degrees Celsius.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
A thermodynamic engine with an efficiency of 30% performs 2500 J of work.How much thermal energy is discharged to the lower temperature reservoir?
A)5800 J
B)8300 J
C)750 J
D)1400 J
E)7100 J
A)5800 J
B)8300 J
C)750 J
D)1400 J
E)7100 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
When water at 0°C freezes,the entropy of the water
A)increases.
B)decreases.
C)remains constant.
D)could either increase or decrease; it depends on other factors.
A)increases.
B)decreases.
C)remains constant.
D)could either increase or decrease; it depends on other factors.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
A thermodynamic engine absorbs 85.6 kJ of energy each cycle and exhausts 61.8 kJ.
(a)What is the efficiency of the engine?
(b)How much work does it do each cycle?
(a)What is the efficiency of the engine?
(b)How much work does it do each cycle?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
A thermodynamic engine has an efficiency of 31.4% and receives 8.72 kJ of energy per cycle.
(a)How much work does it perform in each cycle?
(b)How much energy does it exhaust in each cycle?
(a)How much work does it perform in each cycle?
(b)How much energy does it exhaust in each cycle?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
During each cycle,a thermodynamic engine takes in 4.0 J of energy,does 1.0 J of work,and expels 3.0 J of energy.What is its efficiency?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
What is the efficiency of an ideal Carnot engine operating between a reservoir in which ice and water coexist,and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atm for both reservoirs.
A)27%
B)0.27%
C)100%
D)1.0%
E)15%
A)27%
B)0.27%
C)100%
D)1.0%
E)15%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
For a certain ideal Carnot engine,the hot reservoir is 35 C° higher than the cold reservoir.If this engine is to have an efficiency of 20%,what must be the temperature of the hot reservoir?
A)70.0 K
B)140 K
C)175 K
D)210 K
E)245 K
A)70.0 K
B)140 K
C)175 K
D)210 K
E)245 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
Two ideal Carnot thermodynamic engines have the same efficiency.One operates between 5.0 × 102 K and 3.0 × 102 K,and the other between 4.0 × 102 K and some lower temperature.What is the lower temperature?
A)200 K
B)220 K
C)240 K
D)260 K
E)280 K
A)200 K
B)220 K
C)240 K
D)260 K
E)280 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
An inventor tries to sell you his new thermodynamic engine that takes in 40 J of energy at 87°C on each cycle,expels 30 J at 27°C,and does 10 J of work.Would it be wise to invest in this engine? Back up your conclusion with numerical calculations.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
One of the most efficient engines built so far has the following characteristics: The combustion chamber temperature is 1900°C,the exhaust temperature = 430°C,7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour.(1 cal = 4.186 J)
(a)What is the actual efficiency of this engine?
(b)What is the power output of this engine?
(c)What would be the maximum possible efficiency for an engine using the same temperature extremes?
(a)What is the actual efficiency of this engine?
(b)What is the power output of this engine?
(c)What would be the maximum possible efficiency for an engine using the same temperature extremes?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
A real (non-Carnot)thermodynamic engine,operating between thermal energy reservoirs at temperatures of 450 K and 270 K,performs 3.3 kJ of net work,and rejects 8.2 kJ of energy in a single cycle.
(a)What is the thermal efficiency of this thermodynamic engine?
(b)What is the maximum efficiency it could possibly have?
(a)What is the thermal efficiency of this thermodynamic engine?
(b)What is the maximum efficiency it could possibly have?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
An ideal Carnot engine has an efficiency of 83.0% and performs 4500 J of work every cycle.How much energy is discharged to the lower temperature reservoir every cycle?
A)920 J
B)830 J
C)740 J
D)3700 J
E)5400 J
A)920 J
B)830 J
C)740 J
D)3700 J
E)5400 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
An ideal Carnot engine operating between a warm reservoir of unknown temperature and a cold reservoir at 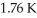 has an efficiency of
has an efficiency of 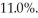 What is the temperature of the warm reservoir?
What is the temperature of the warm reservoir?
A)1.98 K
B)0.180 K
C)157 K
D)0.160 K
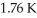 has an efficiency of
has an efficiency of 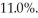 What is the temperature of the warm reservoir?
What is the temperature of the warm reservoir?A)1.98 K
B)0.180 K
C)157 K
D)0.160 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
An ideal Carnot engine operates between a high temperature reservoir at 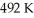 and a river with water at
and a river with water at 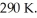 If it absorbs
If it absorbs 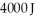 of energy each cycle,how much work per cycle does it perform?
of energy each cycle,how much work per cycle does it perform?
A)1642 J
B)2358 J
C)1483 J
D)2517 J
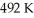 and a river with water at
and a river with water at 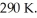 If it absorbs
If it absorbs 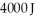 of energy each cycle,how much work per cycle does it perform?
of energy each cycle,how much work per cycle does it perform?A)1642 J
B)2358 J
C)1483 J
D)2517 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
A thermodynamic engine having the maximum possible efficiency has an efficiency of 25% when operating between two heat reservoirs.If the temperature of the cold reservoir is 300 K,what is the temperature of the hot reservoir?
A)350 K
B)375 K
C)400 K
D)450 K
E)500 K
A)350 K
B)375 K
C)400 K
D)450 K
E)500 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
A thermodynamic engine having the maximum possible efficiency has an efficiency of 35.0% when operating between two heat reservoirs.If the temperature of the hot reservoir is 700 K,what is the temperature of the cold reservoir?
A)200 K
B)245 K
C)350 K
D)455 K
E)600 K
A)200 K
B)245 K
C)350 K
D)455 K
E)600 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
A Carnot engine operates between two reservoirs with unknown temperatures.If the Carnot engine operates at 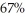 efficiency,what is the ratio of the absolute temperatures of the reservoirs,Tc/Th?
efficiency,what is the ratio of the absolute temperatures of the reservoirs,Tc/Th?
A)0.33
B)0.0012
C)0.0025
D)0.67
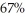 efficiency,what is the ratio of the absolute temperatures of the reservoirs,Tc/Th?
efficiency,what is the ratio of the absolute temperatures of the reservoirs,Tc/Th?A)0.33
B)0.0012
C)0.0025
D)0.67

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
The "hot shot" thermodynamic engine operating between 40°C and 380°C has an efficiency that is 60% of that of an ideal Carnot engine operating between the same temperatures.If the "hot shot" engine absorbs energy at a rate of 60 kW,at what rate does it exhaust energy?
A)36 kW
B)41 kW
C)57 kW
D)60 kW
A)36 kW
B)41 kW
C)57 kW
D)60 kW

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
The ocean thermal energy conversion project uses the surface water near tropical islands with a temperature of 20°C as the hot temperature reservoir,and the water at some depth,with a temperature of 5.0°C,as the cold temperature reservoir for a thermodynamic engine.What is the maximum possible efficiency of an engine running between those two temperatures?
A)4.7%
B)5.1%
C)7.9%
D)15%
E)30%
A)4.7%
B)5.1%
C)7.9%
D)15%
E)30%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
A coal-fired plant generates 600 MW of electric power.The plant uses 4.8 × 106 kg of coal each day,and the heat of combustion of coal is 3.3 × 107 J/kg.The steam that drives the turbines is at a temperature of 300°C,and the exhaust water is at 37°C.
(a)What is the overall efficiency of the plant for generating electric power?
(b)How much thermal energy is exhausted each day?
(c)Using the same heat reservoirs,what is the maximum possible efficiency for a thermodynamic engine?
(a)What is the overall efficiency of the plant for generating electric power?
(b)How much thermal energy is exhausted each day?
(c)Using the same heat reservoirs,what is the maximum possible efficiency for a thermodynamic engine?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
An ideal Carnot thermodynamic engine has an efficiency of 0.600.If it operates between a deep lake with a constant temperature of 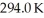 and a hot reservoir,what is the temperature of the hot reservoir?
and a hot reservoir,what is the temperature of the hot reservoir?
A)735 K
B)490 K
C)470 K
D)784 K
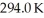 and a hot reservoir,what is the temperature of the hot reservoir?
and a hot reservoir,what is the temperature of the hot reservoir?A)735 K
B)490 K
C)470 K
D)784 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
An ideal Carnot thermodynamic engine operates between 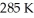 and
and 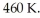 What is its efficiency?
What is its efficiency?
A)0.38
B)0.62
C)0.61
D)1.61
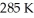 and
and 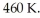 What is its efficiency?
What is its efficiency?A)0.38
B)0.62
C)0.61
D)1.61

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
An ideal Carnot engine operating between a warm reservoir of unknown temperature and a cold reservoir at 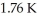 has an efficiency of
has an efficiency of 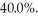 What is the temperature of the warm reservoir?
What is the temperature of the warm reservoir?
A)2.93 K
B)0.0500 K
C)106 K
D)0.0400 K
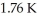 has an efficiency of
has an efficiency of 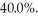 What is the temperature of the warm reservoir?
What is the temperature of the warm reservoir?A)2.93 K
B)0.0500 K
C)106 K
D)0.0400 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
An ideal Carnot engine operates between a warm reservoir at 233 K and a colder reservoir.During each cycle,this engine extracts 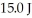 of energy from the warm reservoir and does
of energy from the warm reservoir and does 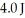 of work.What is the temperature of the colder reservoir?
of work.What is the temperature of the colder reservoir?
A)171 K
B)62 K
C)47 K
D)67 K
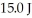 of energy from the warm reservoir and does
of energy from the warm reservoir and does 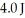 of work.What is the temperature of the colder reservoir?
of work.What is the temperature of the colder reservoir?A)171 K
B)62 K
C)47 K
D)67 K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
An ideal Carnot engine extracts 529 J of energy from a high-temperature reservoir during each cycle,and rejects 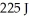 of energy to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?
of energy to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?
A)0.57
B)1.35
C)2.35
D)0.7
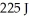 of energy to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?
of energy to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?A)0.57
B)1.35
C)2.35
D)0.7

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
An ideal Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C.How much energy does it use to remove 2000 J of thermal energy from the interior of the house?
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
A reversible engine takes in energy at a rate of 555 W and exhausts energy at 482 W.
(a)How much power does it produce?
(b)What is its efficiency?
(c)If operated in reverse as a refrigerator,what would be its performance coefficient (COP)?
(a)How much power does it produce?
(b)What is its efficiency?
(c)If operated in reverse as a refrigerator,what would be its performance coefficient (COP)?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
During each cycle,the compressor in a certain ideal Carnot refrigerator performs 480 J of work to remove 150 J of thermal energy from the interior of the refrigerator.How much thermal energy do the coils behind the refrigerator discharge into the kitchen each cycle?
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
A refrigerator has a performance coefficient (COP)of 1.15,and it extracts 7.95 J of energy from the cold reservoir during each cycle.
(a)How much work is done on the gas in each cycle?
(b)How much energy is exhausted into the hot reservoir in each cycle?
(a)How much work is done on the gas in each cycle?
(b)How much energy is exhausted into the hot reservoir in each cycle?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
A thermodynamic pump absorbs thermal energy from the atmosphere at a rate of 30 kW.If work is being done to run this thermodynamic pump at a rate of 7.7 kW,what is the coefficient of performance (COP)of the thermodynamic pump?
A)3.9
B)4.9
C)2.9
D)0.26
E)22
A)3.9
B)4.9
C)2.9
D)0.26
E)22

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
A Carnot air conditioner has a coefficient of performance of 17.0 and removes 72.0 MJ of thermal energy from the interior of a house every hour.How much power does it consume?
A)1180 W
B)1320 W
C)520 kW
D)3.14 MW
E)1.25 MW
A)1180 W
B)1320 W
C)520 kW
D)3.14 MW
E)1.25 MW

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
What is the change in entropy when 15.0 g of water at 100°C are turned into steam at 100°C? The latent heat of vaporization of water is 22.6 × 105 J/kg.
A)90.8 J/K
B)-90.8 J/K
C)339 J/K
D)-339 J/K
E)0 J/K
A)90.8 J/K
B)-90.8 J/K
C)339 J/K
D)-339 J/K
E)0 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
A 15.0-kg block of ice at 0.00°C falls into Lake Superior,which is a fresh water lake.The lake water is at 10.0°C,and the latent heat of fusion for ice is 3.34 × 105 J/kg.Find the change in entropy due to the melting of the ice (a)of the block of ice,(b)of the lake,and (c)of the system consisting of the lake and the ice.(d)Is your answer to part (c)consistent with the reversibility or irreversibility of the melting process?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
During each cycle of operation,a refrigerator absorbs 230 J of energy from the freezer and expels 356 J of energy to the room.How much work input is required in each cycle?
A)712 J
B)586 J
C)460 J
D)126 J
A)712 J
B)586 J
C)460 J
D)126 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
An air conditioner with a coefficient of performance of 3.50 uses 30.0 kW of power to operate.What power is it discharging to the outdoors?
A)30.0 kW
B)75.0 kW
C)105 kW
D)135 kW
E)210 kW
A)30.0 kW
B)75.0 kW
C)105 kW
D)135 kW
E)210 kW

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
An ideal Carnot engine is operated as a thermodynamic pump to heat a room in the winter.The thermodynamic pump delivers thermal energy to the room at the rate of 47 kJ per second and maintains the room at a temperature of 293 K when the outside temperature is 237 K.The power requirement to run the thermodynamic pump under these operating conditions is closest to
A)9000 W.
B)7100 W.
C)20,000 W.
D)15,000 W.
E)11,000 W.
A)9000 W.
B)7100 W.
C)20,000 W.
D)15,000 W.
E)11,000 W.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
Suppose that the Department of Energy develops a new reversible engine that has a coefficient of performance (COP)of 4.0 when operated as a refrigerator and a COP of 5.0 when operated as a thermodynamic pump.What is its thermal efficiency when operated as a thermodynamic engine doing work?
A)10%
B)20%
C)25%
D)45%
E)80%
A)10%
B)20%
C)25%
D)45%
E)80%

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
An ideal Carnot refrigerator powered from an electrical outlet takes heat from water at 0.0°C and rejects energy to a room at 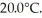 How much electrical energy must be supplied to the refrigerator so it will convert 78 g of water at 0.0°C to ice at 0.0°C.The latent heat of fusion of water is 3.34 × 105 J/kg.
How much electrical energy must be supplied to the refrigerator so it will convert 78 g of water at 0.0°C to ice at 0.0°C.The latent heat of fusion of water is 3.34 × 105 J/kg.
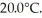 How much electrical energy must be supplied to the refrigerator so it will convert 78 g of water at 0.0°C to ice at 0.0°C.The latent heat of fusion of water is 3.34 × 105 J/kg.
How much electrical energy must be supplied to the refrigerator so it will convert 78 g of water at 0.0°C to ice at 0.0°C.The latent heat of fusion of water is 3.34 × 105 J/kg.
Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
During each cycle,a refrigerator removes 20.0 kJ of heat from the freezing compartment and ejects 24.0 kJ into a room.
(a)How much work per cycle is required each cycle to run this refrigerator?
(b)What is the coefficient of performance of this refrigerator?
(a)How much work per cycle is required each cycle to run this refrigerator?
(b)What is the coefficient of performance of this refrigerator?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
An ideal Carnot engine is operated as an air conditioner to cool a house in the summer.The air conditioner removes 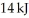 of thermal energy per second from the house,and maintains the inside temperature at
of thermal energy per second from the house,and maintains the inside temperature at 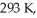 while the outside temperature is
while the outside temperature is 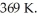 The power required to run the air conditioner under these operating conditions is closest to
The power required to run the air conditioner under these operating conditions is closest to
A)3600 W.
B)4400 W.
C)5100 W.
D)5800 W.
E)6600 W.
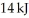 of thermal energy per second from the house,and maintains the inside temperature at
of thermal energy per second from the house,and maintains the inside temperature at 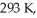 while the outside temperature is
while the outside temperature is 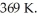 The power required to run the air conditioner under these operating conditions is closest to
The power required to run the air conditioner under these operating conditions is closest toA)3600 W.
B)4400 W.
C)5100 W.
D)5800 W.
E)6600 W.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
If the efficiency of a reversible engine is 28%,what is its COP (performance coefficient)operated (a)as a refrigerator,and (b)as a thermodynamic pump?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
An ideal reversible thermodynamic pump is taking heat from the outside air at -10.0°C and discharging it into the house at 18.0°C.What is the coefficient of performance of this thermodynamic pump?
A)10.4
B)9.44
C)0.644
D)0.533
E)0.0962
A)10.4
B)9.44
C)0.644
D)0.533
E)0.0962

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
A thermodynamic pump with a performance coefficient (COP)of 4.9 absorbs thermal energy from the atmosphere at a rate of 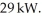 At what rate is work being done to run this thermodynamic pump?
At what rate is work being done to run this thermodynamic pump?
A)6 kW
B)142 kW
C)113 kW
D)35 kW
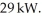 At what rate is work being done to run this thermodynamic pump?
At what rate is work being done to run this thermodynamic pump?A)6 kW
B)142 kW
C)113 kW
D)35 kW

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
A refrigerator has a coefficient of performance equal to 4.2.How much work must be done on the operating gas in the refrigerator in order to remove 250 J of energy from the interior compartment?
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
An ideal Carnot refrigerator with a performance coefficient (COP)of 5.0 cools items inside of it to 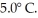 What is the high temperature needed to operate this refrigerator?
What is the high temperature needed to operate this refrigerator?
A)61° C
B)1395° C
C)6° C
D)30° C
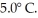 What is the high temperature needed to operate this refrigerator?
What is the high temperature needed to operate this refrigerator?A)61° C
B)1395° C
C)6° C
D)30° C

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
When 1.0 kg of steam at 100°C condenses to water at 100°C,what is the change in entropy of the steam? The latent heat of vaporization of water is 22.6 × 105 J/kg.
A)zero
B)6.1 × 103 J/K
C)-6.1 × 103 J/K
D)22.6 × 105 J/K
E)-22.6 × 105 J/K
A)zero
B)6.1 × 103 J/K
C)-6.1 × 103 J/K
D)22.6 × 105 J/K
E)-22.6 × 105 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
What is the change in entropy of the ice when a 0.050-kg ice cube at 0°C melts? The latent heat of fusion for water is 80 cal/g.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
When 0.50 kg of water at 0°C freezes,what is the change in entropy of the water? The latent heat of fusion of water is 33,400 J/kg.
A)0 J/K
B)610 J/K
C)-610 J/K
D)-17,000 J/K
E)17,000 J/K
A)0 J/K
B)610 J/K
C)-610 J/K
D)-17,000 J/K
E)17,000 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
What is the entropy change of 450 g of water when it changes from (a)liquid to steam at its usual boiling point and (b)ice to liquid at its usual melting point? For water,LF = 0.334 MJ/kg,LV = 2.26 MJ/kg.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
On a cold winter day,the outside temperature is -20°C and the inside temperature is maintained at 20°C.There is a net thermal energy flow to the outside through the walls,roof,etc.,of 25 kW.What is the rate of increase of the entropy of the universe as a result of this energy flow?
A)13 W/K
B)25 W/K
C)85 W/K
D)99 W/K
E)0 W/K
A)13 W/K
B)25 W/K
C)85 W/K
D)99 W/K
E)0 W/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
A 0.42-kg quantity of ethanol,in the liquid state at its melting point of -114.4°C,is frozen at atmospheric pressure.The heat of fusion of ethanol is 1.04 × 105 J/kg,and its molecular mass is 46.1 g/mol.What is the change in the entropy of the ethanol as it freezes?
A)-280 J/K
B)-250 J/K
C)-310 J/K
D)250 J/K
E)280 J/K
A)-280 J/K
B)-250 J/K
C)-310 J/K
D)250 J/K
E)280 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
What is the change in entropy of the lead when 2.0 kg of molten lead at its melting point temperature solidifies? For lead: LV = 207 kcal/kg at 1744°C,LF = 5.9 kcal/kg at 328°C.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
A container of ideal gas at STP undergoes an isothermal expansion and its entropy changes by 3.7 J/K.How much work does it do?
A)0.0 J
B)1.0 × 103 J
C)-1.0 × 103 J
D)1.4 × 103 J
E)-1.4 × 103 J
A)0.0 J
B)1.0 × 103 J
C)-1.0 × 103 J
D)1.4 × 103 J
E)-1.4 × 103 J

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
An irreversible engine operating between the temperatures of 550 K and 300 K extracts 1.20 kJ of thermal energy from the hot reservoir and produces 0.450 kJ of work.How much entropy is created in the process?
A)0.32 J/K
B)0.68 J/K
C)0.44 J/K
D)0.73 J/K
E)0 J/K
A)0.32 J/K
B)0.68 J/K
C)0.44 J/K
D)0.73 J/K
E)0 J/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
On a cold winter day,the outside temperature is -20°C and the inside temperature is maintained at 20°C.There is a net thermal energy flow to the outside through the walls,roof,etc.,of 25 kW.At what rate is the entropy of the air outside the house changing as a result of this process?
A)+13 W/K
B)-25 W/K
C)85 W/K
D)+99 W/K
E)0 W/K
A)+13 W/K
B)-25 W/K
C)85 W/K
D)+99 W/K
E)0 W/K

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


