Deck 4: The Modern Model of the Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/103
Play
Full screen (f)
Deck 4: The Modern Model of the Atom
1
Which of the following species does not match the electronic configuration 1s22s22p63s23p64s23d104p6 ?
A)Br-
B)Sr+2
C)As-3
D)Se+2
A)Br-
B)Sr+2
C)As-3
D)Se+2
Se+2
2
For the electron of a hydrogen atom, which of the following quantum "jumps" requires the most energy?
A)from n = 3 to n = 4
B)from n = 2 to n = 3
C)from n = 4 to n = 5
D)from n = 1 to n = 2
A)from n = 3 to n = 4
B)from n = 2 to n = 3
C)from n = 4 to n = 5
D)from n = 1 to n = 2
from n = 1 to n = 2
3
Which of the following anions will have the electronic configuration 1s22s22p63s23p6 ?
A)K1-
B)Cl1-
C)S2-
D)Br1-
A)K1-
B)Cl1-
C)S2-
D)Br1-
Cl1-
4
Which of the following cations will have the electronic configuration 1s22s22p63s23p6 ?
A)Co2+
B)Ca2+
C)Sc1+
D)Cl1+
A)Co2+
B)Ca2+
C)Sc1+
D)Cl1+

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following subshells does not appear in the third shell?
A)s
B)p
C)d
D)f
A)s
B)p
C)d
D)f

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following elements never obeys the octet rule?
A)Hydrogen
B)Nitrogen
C)Carbon
D)Chlorine
A)Hydrogen
B)Nitrogen
C)Carbon
D)Chlorine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
7
How many electrons can be "stored" in a completely filled p-block of elements?
A)10
B)3
C)6
D)5
A)10
B)3
C)6
D)5

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
8
A non-metal is an element that tends to ________ valence electrons in chemical reactions, becoming a(n)________ in the process.
A)lose; cation
B)gain; anion
C)lose; anion
D)gain; cation
A)lose; cation
B)gain; anion
C)lose; anion
D)gain; cation

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
9
The likely formula of the compound formed between Al and S is ________.
A)Al2S
B)AlS2
C)Al2S3
D)Al3S2
A)Al2S
B)AlS2
C)Al2S3
D)Al3S2

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
10
A metal is an element that tends to ________ valence electrons in chemical reactions, becoming a(n)________ in the process.
A)lose; cation
B)gain; anion
C)lose; anion
D)gain; cation
A)lose; cation
B)gain; anion
C)lose; anion
D)gain; cation

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
11
The correct order of increasing energy in the following subshells is:
A)2p, 3s, 4s, 3p, 3d
B)2p, 3s, 3p, 4s, 3d
C)2p, 3s, 3p, 3d, 4s
D)2p, 3s, 3d, 3p, 4s
A)2p, 3s, 4s, 3p, 3d
B)2p, 3s, 3p, 4s, 3d
C)2p, 3s, 3p, 3d, 4s
D)2p, 3s, 3d, 3p, 4s

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
12
Which is the correct electronic configuration for Fe (atomic number 26)in the ground state?
A)1s22s22p63s23p64s23d6
B)1s22s22p63s23p63d8
C)1s22s22p63s23p63d64s2
D)1s22s22p63s23p64s24p6
A)1s22s22p63s23p64s23d6
B)1s22s22p63s23p63d8
C)1s22s22p63s23p63d64s2
D)1s22s22p63s23p64s24p6

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
13
The likely formula of the compound formed between Ca and O is ________.
A)Ca2O
B)CaO2
C)Ca2O3
D)CaO
A)Ca2O
B)CaO2
C)Ca2O3
D)CaO

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following subshells appear in the second shell?
A)s
B)s,p
C)s, p, d
D)p
A)s
B)s,p
C)s, p, d
D)p

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
15
Which species has the following electronic configuration: 1s22s22p63s23p6 ?
A)F-
B)Mg+2
C)P-3
D)S-
A)F-
B)Mg+2
C)P-3
D)S-

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
16
How many electrons can be "stored" in a completely filled 4f sublevel?
A)14
B)10
C)7
D)28
A)14
B)10
C)7
D)28

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
17
The total number of electrons that can be accommodated in 3d is ________.
A)5
B)2
C)6
D)10
A)5
B)2
C)6
D)10

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
18
Which is the correct electronic configuration for Se (atomic number 34)in the ground state?
A)1s22s22p63s23p63d104s24p4
B)1s22s22p63s23p63d104s04p6
C)1s22s22p63s23p64s23d104p4
D)1s22s22p63s23p63d104s03p6
A)1s22s22p63s23p63d104s24p4
B)1s22s22p63s23p63d104s04p6
C)1s22s22p63s23p64s23d104p4
D)1s22s22p63s23p63d104s03p6

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
19
Which feature of Bohr's atomic model is no longer accepted as true by today's scientists?
A)that most of an atom's mass is located within the nucleus
B)that electrons have only certain, allowed energy values
C)that electrons orbit the nucleus in circular paths
D)that each principal quantum level can hold a maximum of 2n2 electrons
A)that most of an atom's mass is located within the nucleus
B)that electrons have only certain, allowed energy values
C)that electrons orbit the nucleus in circular paths
D)that each principal quantum level can hold a maximum of 2n2 electrons

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
20
What is the main difference between "classical physics" and "quantum physics"?
A)Quantum physics requires the speed of light to be a constant value.
B)Classical physics requires that only certain, allowed values for energy be used.
C)Quantum physics requires that only certain, allowed values for energy be used.
D)Quantum physics can only describe the behavior of very small objects, like atoms and molecules.
A)Quantum physics requires the speed of light to be a constant value.
B)Classical physics requires that only certain, allowed values for energy be used.
C)Quantum physics requires that only certain, allowed values for energy be used.
D)Quantum physics can only describe the behavior of very small objects, like atoms and molecules.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
21
Which orbital is dumbbell-shaped?
A)2s
B)3p
C)4d
D)5g
A)2s
B)3p
C)4d
D)5g

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
22
Which species has the following electronic configuration: 1s22s22p63s23p3 ?
A)Al
B)P
C)S
D)Si
A)Al
B)P
C)S
D)Si

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
23
Atoms in which of the following groups will likely gain three electrons?
A)IIIA
B)VA
C)VIIA
D)both A and B
A)IIIA
B)VA
C)VIIA
D)both A and B

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following does not have the neon electron configuration?
A)S4+
B)Mg2+
C)O2-
D)Al3+
A)S4+
B)Mg2+
C)O2-
D)Al3+

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
25
Which color light is composed of photons having the largest energy?
A)blue
B)green
C)yellow
D)red
A)blue
B)green
C)yellow
D)red

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
26
The valence shell ________.
A)always contains eight electrons
B)is the lowest energy shell in an atom
C)is the outermost occupied shell in an atom
D)is always the same for anions and cations
A)always contains eight electrons
B)is the lowest energy shell in an atom
C)is the outermost occupied shell in an atom
D)is always the same for anions and cations

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
27
Which orbital is a spherical shape?
A)2s
B)3p
C)4d
D)5f
A)2s
B)3p
C)4d
D)5f

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
28
The ion that has 14 protons and 18 electrons is ________.
A)Si4+
B)Si4-
C)N4+
D)N4-
A)Si4+
B)Si4-
C)N4+
D)N4-

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following does not include [Kr] in its shorthand electron configuration?
A)Sr2+
B)Te2-
C)Se6+
D)Mo
A)Sr2+
B)Te2-
C)Se6+
D)Mo

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following has attained a noble gas electronic configuration?
A)Si4+
B)Cl1+
C)Al3-
D)Cr3+
A)Si4+
B)Cl1+
C)Al3-
D)Cr3+

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
31
Atoms in which of the following groups will likely lose three electrons?
A)IIIA
B)VA
C)IIA
D)IVA
A)IIIA
B)VA
C)IIA
D)IVA

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
32
What is the number of outer shell electrons in fluorine?
A)2
B)5
C)7
D)9
A)2
B)5
C)7
D)9

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
33
The scientist credited with developing the current mathematical model of the atom is ________.
A)Bohr
B)Schrodinger
C)Planck
D)Rutherford
A)Bohr
B)Schrodinger
C)Planck
D)Rutherford

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
34
How many valence electrons are in silicon?
A)2
B)4
C)5
D)7
A)2
B)4
C)5
D)7

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
35
The size of boron is ________ than that of oxygen and ________ than that of aluminum.
A)smaller; larger
B)larger; larger
C)smaller; smaller
D)larger; smaller
A)smaller; larger
B)larger; larger
C)smaller; smaller
D)larger; smaller

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
36
Which color light is composed of photons having the longest wavelength?
A)blue
B)green
C)yellow
D)red
A)blue
B)green
C)yellow
D)red

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
37
The elements of Group VIIIA are almost entirely non-reactive with any of the other chemical elements. Which of the following statements best explains this observed behavior?
A)Group VIIIA elements comply with the octet rule.
B)Group VIIIA elements are so rare that no one has ever studied their reactive properties in any detail.
C)Group VIIIA elements are all gases, and gases are generally non-reactive.
D)No explanation given here can explain why Group VIIIA elements do not react with any of the other chemical elements.
A)Group VIIIA elements comply with the octet rule.
B)Group VIIIA elements are so rare that no one has ever studied their reactive properties in any detail.
C)Group VIIIA elements are all gases, and gases are generally non-reactive.
D)No explanation given here can explain why Group VIIIA elements do not react with any of the other chemical elements.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
38
Below are four pairs of atoms and ions. In which pair is the larger of the two species written first?
A)S2- and S
B)Cu2+ and Cu
C)O and O2-
D)Cr3+ and Cr
A)S2- and S
B)Cu2+ and Cu
C)O and O2-
D)Cr3+ and Cr

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
39
The relative ratio of atoms in the binary compound AlxOy is ________.
A)x=2, y=3
B)x=3, y=2
C)x=5, y=2
D)x=2, y=5
A)x=2, y=3
B)x=3, y=2
C)x=5, y=2
D)x=2, y=5

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
40
Which type of electromagnetic radiation is composed of photons having the largest energy?
A)microwaves
B)radio waves
C)x rays
D)gamma rays
A)microwaves
B)radio waves
C)x rays
D)gamma rays

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following elements is the smallest size chalcogen?
A)H
B)N
C)O
D)F
A)H
B)N
C)O
D)F

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
42
Which s orbital is the largest?
A)1s
B)2s
C)-2s
D)-1s
A)1s
B)2s
C)-2s
D)-1s

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the energy in joules of microwave radiation of wavelength 12.7 centimeters. (E = hν, h = 6.63 × 10-34 J sec; speed of light = wavelength × ν, speed of light = 3 × 108 meters/sec.)
A)4.87 × 1019 J
B)7.30 × 10-19 J
C)3.61 × 10-18 J
D)1.56 × 10-24 J
A)4.87 × 1019 J
B)7.30 × 10-19 J
C)3.61 × 10-18 J
D)1.56 × 10-24 J

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
44
The only possible way for an excited state electron to return to the ground state is to emit energy in the form of visible light.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
45
The units of h (Planck's constant)are ________.
A)J.s
B)J/s
C)m/s
D)J/nm
A)J.s
B)J/s
C)m/s
D)J/nm

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
46
The p-block represents a region on the periodic table which is 6 elements in width.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the formula of the compound produced in a reaction between barium (Ba)and oxygen (O).
A)(BaO2)
B)BaO
C)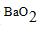
D)(Ba2O3)
A)(BaO2)
B)BaO
C)
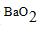
D)(Ba2O3)

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
48
What is the wavelength (in meters)of radio waves that have a frequency of 89.9 MHz? (wavelength × frequency = speed of light; speed of light = 3 × 108 meters/sec.)
A)3.34 centimeters
B)2.70 × 1016 millimeters
C)0.300 meters
D)3.34 meters
A)3.34 centimeters
B)2.70 × 1016 millimeters
C)0.300 meters
D)3.34 meters

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
49
The s-type orbitals of any given principal energy level are always spherical in shape.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
50
The f-block represents a region on the periodic table which is 10 elements in width.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
51
Metals generally become more reactive as one proceeds down a column (group).

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
52
Atoms tend to increase in size as one moves from left to right across a period.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following elements is the largest size halogen?
A)He
B)Br
C)F
D)Kr
A)He
B)Br
C)F
D)Kr

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
54
What is the trend in atomic size in the series of Group IIA elements Be through Ra?
A)Atomic size increases in this series.
B)Atomic size decreases in this series.
C)Atomic size remains unchanged in this series.
D)Atomic size increases at first, then decreases in this series.
A)Atomic size increases in this series.
B)Atomic size decreases in this series.
C)Atomic size remains unchanged in this series.
D)Atomic size increases at first, then decreases in this series.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
55
In a single atom, what is the maximum number of electrons that can have quantum number n=4?
A)10
B)14
C)18
D)32
A)10
B)14
C)18
D)32

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
56
Bohr's atomic theory places an atom's electrons in discrete-energy circular paths around a central, positively charged nucleus.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
57
Phosphorus atoms have five valence electrons.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
58
Predict the formula of the compound produced in a reaction between lithium (Li)and phosphorus (P).
A)LiP
B)(Li2P3)
C)(LiP2)
D)(Li3P)
A)LiP
B)(Li2P3)
C)(LiP2)
D)(Li3P)

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
59
The observed line spectra of electrically excited gas samples tend to support the notion that electron energies are quantized.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
60
The n = 5 principal energy level can hold a maximum of 25 electrons.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
61
The atomic radius can be calculated by x-ray diffraction.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
62
Halogens have large electron affinity values.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
63
Modern atomic theory places electrons into complex mathematical probability functions called "orbitals."

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
64
The correct shortcut for writing the electronic configuration of Sr is [Ar]4s2.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
65
The atom size decreases as one proceeds from left to right across a period.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
66
H+ has one electron.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
67
An electron in the excited state does not necessarily return to a ground state in a single jump.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
68
The element is defined by its atomic number.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
69
The 95% boundary indicates the area where 95% of the time the electron may be found.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
70
If an element has a small electron affinity value it can easily lose an electron.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
71
The wavelength is the distance measured between two crests, but not between two troughs.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
72
+1 is the only charge sodium ions normally have.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
73
The speed of light is about 186,000 miles per minute.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
74
Carbon can assume both +4 and -4 charges.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
75
A non-metal is an element that tends to gain valence electrons in chemical reactions becoming a cation in the process.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
76
Once an atom gets excited, it may remain in that excited state indefinitely.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
77
Modern scientists accept the notion that electrons are found in circular orbits around a central, positively charged nucleus.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
78
The number of electrons in the valence shell of an atom is equal to the group number for the representative elements.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
79
Should an oxygen atom (O)gain two electrons, it would then have the same ground state electronic configuration as an atom of neon (Ne), thus satisfying the octet rule.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
80
Shells get larger as the principal quantum number n increases.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck


