Deck 7: Intermolecular Forces and the Phases of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Premises:
sodium oxide
sodium oxide
water
water
Responses:
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
Question
Match between columns
Premises:
quartz
quartz
quartz
quartz
iron
iron
iron
iron
carbon tetrabromide
carbon tetrabromide
carbon tetrabromide
carbon tetrabromide
water
water
water
water
Responses:
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
Question
Match between columns
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/71
Play
Full screen (f)
Deck 7: Intermolecular Forces and the Phases of Matter
1
Which of the three phases of matter (solid, liquid, or gas)has particles moving at the highest average velocity?
A)solid
B)liquid
C)gas
D)The particles in all three phases have about the same average speed.
A)solid
B)liquid
C)gas
D)The particles in all three phases have about the same average speed.
gas
2
Between individual molecules of NO2 in the solid state, which of the following types of intermolecular forces would you expect to be dominant?
A)hydrogen bonding
B)London forces
C)van der Waals forces
D)dipole forces
A)hydrogen bonding
B)London forces
C)van der Waals forces
D)dipole forces
dipole forces
3
Which of the following chemical substances would you expect to have the highest melting point?
A)water, H2O
B)methyl alcohol, CH3OH
C)nitrogen dioxide, NO2
D)hydrogen sulfide, H2S
A)water, H2O
B)methyl alcohol, CH3OH
C)nitrogen dioxide, NO2
D)hydrogen sulfide, H2S
water, H2O
4
Which of the three phases of matter (solid, liquid, or gas)features particles in a fixed, rigid arrangement?
A)solid
B)liquid
C)gas
D)All three phases of matter have rigid arrangements of particles.
A)solid
B)liquid
C)gas
D)All three phases of matter have rigid arrangements of particles.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following sublimes?
A)sugar
B)iron
C)mothballs
D)margarine
A)sugar
B)iron
C)mothballs
D)margarine

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the three phases of matter (solid, liquid, or gas)has particles that are in a loose, changeable arrangement but do not completely fill the volume of a given container?
A)solid
B)liquid
C)gas
D)All of the above feature loose arrangements of particles.
A)solid
B)liquid
C)gas
D)All of the above feature loose arrangements of particles.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following chemical substances would you expect to exhibit a degree of hydrogen bonding?
A)NH3
B)CH3OH
C)HF
D)all of the above
A)NH3
B)CH3OH
C)HF
D)all of the above

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
8
The solid to gas conversion is called ________.
A)melting
B)sublimation
C)evaporation
D)condensation
A)melting
B)sublimation
C)evaporation
D)condensation

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the three phases of matter (solid, liquid, or gas)has particles separated by the least amount of distance?
A)solid
B)liquid
C)gas
D)The separation distance is about the same in all three phases.
A)solid
B)liquid
C)gas
D)The separation distance is about the same in all three phases.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
10
The gas to liquid change of state is called ________.
A)sublimation
B)condensation
C)evaporation
D)melting
A)sublimation
B)condensation
C)evaporation
D)melting

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
11
The term "normal" boiling point indicates ________.
A)the temperature at which water evaporates under the atmospheric pressure found at sea level.
B)the temperature at which tap water boils under the atmospheric pressure found at sea level.
C)the temperature at which water boils under any pressure conditions
D)both A and B
A)the temperature at which water evaporates under the atmospheric pressure found at sea level.
B)the temperature at which tap water boils under the atmospheric pressure found at sea level.
C)the temperature at which water boils under any pressure conditions
D)both A and B

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements is true about intermolecular forces of attraction between molecules?
A)They tend to stick molecules together.
B)Cooling is responsible for the substance's liquefaction.
C)The solidification of a substance usually requires lower temperatures than in the case of liquefaction.
D)All of the above are true.
A)They tend to stick molecules together.
B)Cooling is responsible for the substance's liquefaction.
C)The solidification of a substance usually requires lower temperatures than in the case of liquefaction.
D)All of the above are true.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the three phases of matter (solid, liquid, or gas)features particles that are easily compressible?
A)solid
B)liquid
C)gas
D)All three phases of matter are compressible.
A)solid
B)liquid
C)gas
D)All three phases of matter are compressible.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following chemical substances would you expect to have the lowest boiling point (you may consider molecular mass and the degree of intermolecular forces as primary controlling factors)?
A)CH4
B)CH3CH3
C)CH3CH2CH3
D)CH3CH2CH2CH3
A)CH4
B)CH3CH3
C)CH3CH2CH3
D)CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not a solid at 0 °C?
A)salt
B)carbon dioxide
C)sugar
D)iron
A)salt
B)carbon dioxide
C)sugar
D)iron

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
16
Nonpolar molecules may exhibit ________.
A)London forces
B)hydrogen bonding
C)dipolar forces
D)all of the above
A)London forces
B)hydrogen bonding
C)dipolar forces
D)all of the above

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following chemical substances would you expect to have the highest boiling point (you may consider molecular mass and the degree of intermolecular hydrogen bonding as primary controlling factors)?
A)methane, CH4
B)ammonia, NH3
C)methyl alcohol, CH3OH
D)ethyl alcohol, CH3CH2OH
A)methane, CH4
B)ammonia, NH3
C)methyl alcohol, CH3OH
D)ethyl alcohol, CH3CH2OH

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following does not sublime?
A)mothballs
B)iodine crystals
C)salt
D)dry ice
A)mothballs
B)iodine crystals
C)salt
D)dry ice

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
19
The liquid to gas change of state is called ________.
A)sublimation
B)condensation
C)evaporation
D)melting
A)sublimation
B)condensation
C)evaporation
D)melting

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following substances would you expect to be a liquid at room temperature, assuming that strong intermolecular forces need to be present to be a liquid at room temperature?
A)N2
B)CS2
C)H2
D)O2
A)N2
B)CS2
C)H2
D)O2

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following will have the lowest boiling point?
A)H2S
B)H2Te
C)H2Se
D)H2O
A)H2S
B)H2Te
C)H2Se
D)H2O

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
22
The strength of a hydrogen bond is about ________% the strength of a normal O-H bond
A)1
B)4
C)16
D)50
A)1
B)4
C)16
D)50

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
23
To form hydrogen bonds, molecules must contain ________.
A)an N-H bond
B)an O-H bond
C)an H-F bond
D)at least one of the above mentioned bonds
A)an N-H bond
B)an O-H bond
C)an H-F bond
D)at least one of the above mentioned bonds

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
24
Which intermolecular force is important for the function of the antibiotic vancomycin?
A)London forces
B)van der Waals forces
C)hydrogen bonding
D)dipole forces
A)London forces
B)van der Waals forces
C)hydrogen bonding
D)dipole forces

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
25
Which of these has the highest melting point?
A)diamond
B)iron
C)sodium
D)bromine
A)diamond
B)iron
C)sodium
D)bromine

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following will have the highest boiling point?
A)CH3OCH3
B)CH3CH2OH
C)HOCH2-CH2OH
D)CH3CH3
A)CH3OCH3
B)CH3CH2OH
C)HOCH2-CH2OH
D)CH3CH3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
27
How does vancomycin function to kill bacteria?
A)destroys the bacterial DNA
B)interferes with cell wall construction
C)inhibits protein synthesis
D)prevents bacteria metabolism
A)destroys the bacterial DNA
B)interferes with cell wall construction
C)inhibits protein synthesis
D)prevents bacteria metabolism

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following can be called a non-molecular ionic solid?
A)water
B)sodium chloride
C)methane
D)hydrogen
A)water
B)sodium chloride
C)methane
D)hydrogen

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
29
The melting and freezing points are identical for the same compound.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
30
London forces are extremely weak for ________.
A)nitrogen gas
B)methane gas
C)oxygen gas
D)all of the above
A)nitrogen gas
B)methane gas
C)oxygen gas
D)all of the above

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
31
The force(s)responsible for holding the DNA molecule in a very long twisted zipper is ________.
A)hydrogen bonding
B)London forces
C)dipolar forces
D)interionic forces
A)hydrogen bonding
B)London forces
C)dipolar forces
D)interionic forces

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
32
The main reason why carbon tetrabromide is a solid at room temperature when compared to carbon tetrachloride which is a liquid is ________.
A)carbon tetrabromide is tetrahedral in shape
B)carbon tetrachloride has a higher molecular weight
C)carbon tetrabromide may undergo hydrogen bonding
D)all of the above
A)carbon tetrabromide is tetrahedral in shape
B)carbon tetrachloride has a higher molecular weight
C)carbon tetrabromide may undergo hydrogen bonding
D)all of the above

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following will have the highest boiling point?
A)HI
B)HBr
C)HCl
D)HF
A)HI
B)HBr
C)HCl
D)HF

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following may not be considered an ionic compound?
A)sodium chloride
B)magnesium oxide
C)ammonia
D)potassium bromide
A)sodium chloride
B)magnesium oxide
C)ammonia
D)potassium bromide

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following influences the strength of the intermolecular forces?
A)distance between interacting molecules
B)relative orientation between interacting molecules
C)location of the molecule in a liquid or solid
D)All of the above influence the strength of the intermolecular forces.
A)distance between interacting molecules
B)relative orientation between interacting molecules
C)location of the molecule in a liquid or solid
D)All of the above influence the strength of the intermolecular forces.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
36
The strength of an O-H bond is about equal to ________ kJ/bond
A)16
B)50
C)100
D)400
A)16
B)50
C)100
D)400

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following experiences the strongest London forces?
A)carbon tetrachloride
B)water
C)hydrogen
D)helium
A)carbon tetrachloride
B)water
C)hydrogen
D)helium

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
38
Polar molecules may exhibit ________.
A)London forces
B)hydrogen bonding
C)dipolar forces
D)all of the above
A)London forces
B)hydrogen bonding
C)dipolar forces
D)all of the above

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following intermolecular force is the strongest?
A)London forces
B)van der Waals forces
C)hydrogen bonding
D)dipole forces
A)London forces
B)van der Waals forces
C)hydrogen bonding
D)dipole forces

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following does not have a dipole?
A)carbon tetrachloride
B)acetone
C)ammonia
D)water
A)carbon tetrachloride
B)acetone
C)ammonia
D)water

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
41
Identify the following crystalline solids as ionic, molecular, or metallic.
copper (II)carbonate, CuCO3 (or cupric carbonate
copper (II)carbonate, CuCO3 (or cupric carbonate

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
42
The attractive forces between carbon dioxide molecules are considerably stronger than those found in water molecules.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
43
The acetone molecule has a large dipole because of the electronegativity difference between carbon and oxygen.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the following crystalline solids as ionic, molecular, or metallic.
calcium chloride, CaC12
calcium chloride, CaC12

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
45
At the freezing point the molecules are effectively frozen in place.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
46
The replacement of a single NH group on vancomycin causes the antibiotic to become ineffective.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
47
Identify the following crystalline solids as ionic, molecular, or metallic.
glucose, C6H12O6
glucose, C6H12O6

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
48
London forces become significant for large molecules with many atoms and many electrons.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
49
Vancomycin is the first line of defense against bacteria.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
50
Identify the following crystalline solids as ionic, molecular, or metallic.
chromium, Cr
chromium, Cr

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
51
The reason why quartz has a high melting point is hydrogen bonding.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
52
Based upon the formulas and melting points, decide whether each of the following crystalline substances should be classified as ionic, molecular, metallic or network covalent.
magnesium chloride, MgC12, m.p.: 714 °C
magnesium chloride, MgC12, m.p.: 714 °C

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
53
Based upon the formulas and melting points, decide whether each of the following crystalline substances should be classified as ionic, molecular, metallic or network covalent.
silicon carbide, SiC, m.p.: 2700 °C
silicon carbide, SiC, m.p.: 2700 °C

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
54
Solid carbon dioxide is also known as dry ice.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
55
Quartz and diamond are non-molecular covalent substances.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the following crystalline solids as ionic, molecular, or metallic.
iodine, I2
iodine, I2

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
57
The main reason why water has a much higher boiling point than acetone is the ability of water to undergo hydrogen bonding.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
58
Polar molecules do not exhibit London forces.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
59
The freezing point of dry ice is 0 °C.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
60
The terms "dispersion forces" and "London forces" are interchangeable.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
61
Match between columns
Premises:
sodium oxide
sodium oxide
water
water
Responses:
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular
molecular
non-molecular

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
62
Match between columns
Premises:
quartz
quartz
quartz
quartz
iron
iron
iron
iron
carbon tetrabromide
carbon tetrabromide
carbon tetrabromide
carbon tetrabromide
water
water
water
water
Responses:
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding
hydrogen bonding
London forces
metallic
network covalent bonding

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
63
Match between columns

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
64
Match the description of processes that appear in the questions with the corresponding change of states in the list below. 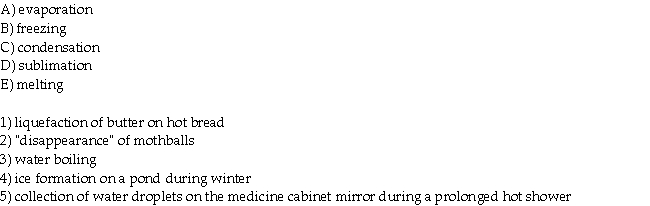


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
64
Based upon the formulas and melting points, decide whether each of the following crystalline substances should be classified as ionic, molecular, metallic or network covalent.
sodium fluoride, NaF, m.p.: 993 °C
sodium fluoride, NaF, m.p.: 993 °C

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
65
Match each temperatures in the questions with the processes described in the list below. 


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
65
Match between columns

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
66
Match each of the substances in the questions with the STRONGEST type of bonding that it exhibits from the list below. 


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
66
What is the general trend in terms of increasing melting point for ionic solids, molecular solids, and network covalent solids?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
67
Identify each of the compounds in the left column as molecular or non-molecular. 


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
67
Based upon the formulas and melting points, decide whether each of the following crystalline substances should be classified as ionic, molecular, metallic or network covalent.
ethanol, C2H6O, m.p.: -117 °C
ethanol, C2H6O, m.p.: -117 °C

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck


