Deck 13: When Reactants Turn Into Products
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 13: When Reactants Turn Into Products
1
Which of the following does not influence the speed of a chemical reaction?
A)concentration of the reactants
B)molecular mass of the reactants
C)surface area of solid reactants
D)temperature
A)concentration of the reactants
B)molecular mass of the reactants
C)surface area of solid reactants
D)temperature
molecular mass of the reactants
2
Which of the following is not a term involved in determining the overall rate of a chemical reaction?
A)the total number of collisions per unit time
B)the total number of collisions in the gas phase
C)the fraction of collisions with energy greater than the activation energy
D)the fraction of collisions which occur with a favorable orientation
A)the total number of collisions per unit time
B)the total number of collisions in the gas phase
C)the fraction of collisions with energy greater than the activation energy
D)the fraction of collisions which occur with a favorable orientation
the total number of collisions in the gas phase
3
Which of the following can be done to increase the likelihood that a chemical reaction will take place?
A)decrease the average speed of the reacting molecules
B)induce more collisions between reacting molecules
C)remove all the water from the reaction mixture
D)break the reaction up into a series of steps
A)decrease the average speed of the reacting molecules
B)induce more collisions between reacting molecules
C)remove all the water from the reaction mixture
D)break the reaction up into a series of steps
induce more collisions between reacting molecules
4
The rate of reaction: A (g)+ 3B (g)→ C (g)+ 2D (g)is Rate = -k[A][B]3. If the concentration of B is doubled while that of A is unchanged, the rate will ________.
A)stay the same
B)be increased by a factor of 6
C)double
D)be increased by a factor of 8
A)stay the same
B)be increased by a factor of 6
C)double
D)be increased by a factor of 8

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
The rate of a reaction is proportional to ________.
A)the total number of collisions that occur per unit time
B)the fraction of collisions with energy larger than the energy of activation
C)the fraction of collisions with the proper orientation
D)All of the above are correct.
A)the total number of collisions that occur per unit time
B)the fraction of collisions with energy larger than the energy of activation
C)the fraction of collisions with the proper orientation
D)All of the above are correct.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an exothermic reaction?
A)burning propane gas in a barbecue
B)burning gasoline when driving a car
C)creating an explosion by detonating nitroglycerine
D)All of the above are exothermic reactions.
A)burning propane gas in a barbecue
B)burning gasoline when driving a car
C)creating an explosion by detonating nitroglycerine
D)All of the above are exothermic reactions.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the reaction X → Y with the following data: Energy of reactants = 50 kJ/mole;
Energy of reaction = -30 kJ/mole;
Energy of transition state = 90 kJ/mole.
The energy of products for the reaction is ________ kJ/mole.
A)-30
B)20
C)30
D)80
Energy of reaction = -30 kJ/mole;
Energy of transition state = 90 kJ/mole.
The energy of products for the reaction is ________ kJ/mole.
A)-30
B)20
C)30
D)80

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8

In the diagram above, the two containers shown (Box A and BoxB)have exactly the same size (volume), are held at the same temperature, and contain reactant molecules (dots). In which box will the rate of reaction be faster?
A)Box A
B)Box B
C)The rate will be about the same in each box.
D)There is no way to tell which will be faster from this diagram.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements regarding reaction mechanisms is true?
A)A mechanism is a series of steps whereby reactants are converted to products.
B)Reaction mechanisms must be determined experimentally.
C)Neither A nor B is true.
D)Both A and B are true.
A)A mechanism is a series of steps whereby reactants are converted to products.
B)Reaction mechanisms must be determined experimentally.
C)Neither A nor B is true.
D)Both A and B are true.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following does not affect the rate of a reaction?
A)the concentration of the reactants
B)the energy of activation
C)the temperature
D)the pressure
A)the concentration of the reactants
B)the energy of activation
C)the temperature
D)the pressure

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the reaction X → Y with the following data: Energy of reactants = 25 kJ/mole;
Energy of products = 75 kJ/mole;
Energy of transition state = 100 kJ/mole.
The energy of activation for the reverse reaction is ________ kJ/mole.
A)25
B)50.
C)75
D)100
Energy of products = 75 kJ/mole;
Energy of transition state = 100 kJ/mole.
The energy of activation for the reverse reaction is ________ kJ/mole.
A)25
B)50.
C)75
D)100

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an endothermic reaction?
A)melting ice
B)freezing water
C)sublimation of dry ice
D)condensing water vapor
A)melting ice
B)freezing water
C)sublimation of dry ice
D)condensing water vapor

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
Which statement is true about the reaction energy profile?
A)plots the relative energies of both the reactants and products
B)plots energy on the y-axis
C)traces the progress of the reaction on the x-axis
D)All of the above are true.
A)plots the relative energies of both the reactants and products
B)plots energy on the y-axis
C)traces the progress of the reaction on the x-axis
D)All of the above are true.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following responses contains the two rate factors that comprise the rate constant (k)for a given chemical reaction?
A)the temperature and the number of collisions per unit volume
B)the number of collisions per unit volume and the fraction of collisions with a favorable orientation
C)the number of collisions with energy greater than the activation energy and the fraction of collisions with a favorable orientation
D)the number of collisions with energy greater than the activation energy and the temperature
A)the temperature and the number of collisions per unit volume
B)the number of collisions per unit volume and the fraction of collisions with a favorable orientation
C)the number of collisions with energy greater than the activation energy and the fraction of collisions with a favorable orientation
D)the number of collisions with energy greater than the activation energy and the temperature

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the reaction C → D with the following data: Energy of reactants = 30 kJ/mole;
Energy of products = 20 kJ/mole;
Energy of transition state = 60 kJ/mole.
One can say with certainty that the reaction is ________ by ________kJ/mole and the energy of activation is ________ kJ/mole.
A)exothermic; 10; 30
B)endothermic; 10; 30
C)exothermic; 40; 30
D)endothermic; 40; 30
Energy of products = 20 kJ/mole;
Energy of transition state = 60 kJ/mole.
One can say with certainty that the reaction is ________ by ________kJ/mole and the energy of activation is ________ kJ/mole.
A)exothermic; 10; 30
B)endothermic; 10; 30
C)exothermic; 40; 30
D)endothermic; 40; 30

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
The units for the rate of a reaction are ________.
A)concentration per unit of time
B)concentration multiplied by the unit of time
C)unit of time per unit of concentration
D)One cannot say with certainty since rate laws vary from reaction to reaction.
A)concentration per unit of time
B)concentration multiplied by the unit of time
C)unit of time per unit of concentration
D)One cannot say with certainty since rate laws vary from reaction to reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following "adjustments" can be made to a chemical reaction system to increase the rate of reaction?
A)increase the reaction temperature
B)increase the concentrations of the reactants
C)add a catalyst
D)All of the above will increase the rate of reaction.
E)None of the above will increase the rate of reaction.
A)increase the reaction temperature
B)increase the concentrations of the reactants
C)add a catalyst
D)All of the above will increase the rate of reaction.
E)None of the above will increase the rate of reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18

In the diagram above, the containers shown (Box A and BoxB)contain exactly the same number of reactant molecules and are at the same temperature. In which box will the rate of reaction be faster?
A)Box A
B)Box B
C)Rate is about the same in each box.
D)No way to tell which is faster from this picture.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an example of a substitution reaction?
A)CH3CH2OH + Br- → CH3CH2Br + OH-
B)2MnO4- + 5C2O42- + 16H+ → 10CO2 + 2Mn2+ + 8H2O
C)2Mg + O2 → 2MgO
D)I2 + H2 → 2HI
A)CH3CH2OH + Br- → CH3CH2Br + OH-
B)2MnO4- + 5C2O42- + 16H+ → 10CO2 + 2Mn2+ + 8H2O
C)2Mg + O2 → 2MgO
D)I2 + H2 → 2HI

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
The fraction of collisions with the proper orientation depends on ________.
A)the strength of the bonds that are to be broken
B)the molecular shape of the molecule
C)the concentration
D)both A and B
A)the strength of the bonds that are to be broken
B)the molecular shape of the molecule
C)the concentration
D)both A and B

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the reaction A + B → Products. If the rate equation is Rate = -k[A]3[B]0 the order of the reaction with respect to A, B, and the total order respectively is ________.
A)3, 1, 1
B)3, 0, 2
C)3, 0, 3
D)3, 1, 4
A)3, 1, 1
B)3, 0, 2
C)3, 0, 3
D)3, 1, 4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following may be considered as biochemical catalysts?
A)carbohydrates
B)DNA
C)enzymes
D)lipids
A)carbohydrates
B)DNA
C)enzymes
D)lipids

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
If the kinetic energy of the reactant molecules is not high enough to overcome the activation energy barrier, ________.
A)the reverse reaction will occur
B)no reaction takes place
C)the reaction will occur anyway
D)the products will form at a slower rate
A)the reverse reaction will occur
B)no reaction takes place
C)the reaction will occur anyway
D)the products will form at a slower rate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
In the following equation 2Y (g)→ Z (g)at 20 °C the following data were obtained: ![<strong>In the following equation 2Y (g)→ Z (g)at 20 °C the following data were obtained: The rate law for this reaction is ________.</strong> A)Rate = -k[Y]<sup>2 </sup> B)Rate = -k[Y] C)Rate = -k[Y]<sup>0</sup> D)Rate = -k[Y]<sup>-</sup><sup>1</sup>](https://storage.examlex.com/TB5644/11ea8554_7386_26b8_9aec_b761bf84aa01_TB5644_00.jpg) The rate law for this reaction is ________.
The rate law for this reaction is ________.
A)Rate = -k[Y]2
B)Rate = -k[Y]
C)Rate = -k[Y]0
D)Rate = -k[Y]-1
![<strong>In the following equation 2Y (g)→ Z (g)at 20 °C the following data were obtained: The rate law for this reaction is ________.</strong> A)Rate = -k[Y]<sup>2 </sup> B)Rate = -k[Y] C)Rate = -k[Y]<sup>0</sup> D)Rate = -k[Y]<sup>-</sup><sup>1</sup>](https://storage.examlex.com/TB5644/11ea8554_7386_26b8_9aec_b761bf84aa01_TB5644_00.jpg) The rate law for this reaction is ________.
The rate law for this reaction is ________.A)Rate = -k[Y]2
B)Rate = -k[Y]
C)Rate = -k[Y]0
D)Rate = -k[Y]-1

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
In the following equation 2 Y (g)+ X (g)→ Z (g)at 100 °C the following data were obtained: ![<strong>In the following equation 2 Y (g)+ X (g)→ Z (g)at 100 °C the following data were obtained: The rate law for this reaction is ________.</strong> A)Rate = k[Y]<sup>2</sup>[X] B)Rate = k[Y][X]<sup>2</sup> C)Rate = k[Y][X] D)Rate = k[Y]<sup>2</sup>[X]<sup>2</sup>](https://storage.examlex.com/TB5644/11ea8554_7385_ffa7_9aec_e1542d7ffdd8_TB5644_00.jpg) The rate law for this reaction is ________.
The rate law for this reaction is ________.
A)Rate = k[Y]2[X]
B)Rate = k[Y][X]2
C)Rate = k[Y][X]
D)Rate = k[Y]2[X]2
![<strong>In the following equation 2 Y (g)+ X (g)→ Z (g)at 100 °C the following data were obtained: The rate law for this reaction is ________.</strong> A)Rate = k[Y]<sup>2</sup>[X] B)Rate = k[Y][X]<sup>2</sup> C)Rate = k[Y][X] D)Rate = k[Y]<sup>2</sup>[X]<sup>2</sup>](https://storage.examlex.com/TB5644/11ea8554_7385_ffa7_9aec_e1542d7ffdd8_TB5644_00.jpg) The rate law for this reaction is ________.
The rate law for this reaction is ________.A)Rate = k[Y]2[X]
B)Rate = k[Y][X]2
C)Rate = k[Y][X]
D)Rate = k[Y]2[X]2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
The difference in energy between the reactants and products in a chemical reaction is called ________.
A)the heat of reaction
B)the activation energy
C)the energy of products
D)either B or C
A)the heat of reaction
B)the activation energy
C)the energy of products
D)either B or C

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is false?
A)The rate determining step is always the slowest step in a multistep mechanism.
B)The rate constant always changes with a variation in temperature.
C)The energy of activation controls the speed of reaction.
D)The rate of a reaction is independent of the concentration of reactants.
A)The rate determining step is always the slowest step in a multistep mechanism.
B)The rate constant always changes with a variation in temperature.
C)The energy of activation controls the speed of reaction.
D)The rate of a reaction is independent of the concentration of reactants.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
In order for a chemical reaction to occur, ________.
A)the reactants must be in sufficient concentration
B)the products must be of lower energy than the reactants
C)the reactants must be of lower energy than the products
D)the reactants must possess sufficient energy to overcome the barrier of the activation energy
A)the reactants must be in sufficient concentration
B)the products must be of lower energy than the reactants
C)the reactants must be of lower energy than the products
D)the reactants must possess sufficient energy to overcome the barrier of the activation energy

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
In the following equation 2W (g)→ V (g)at 20 °C the following data were obtained: ![<strong>In the following equation 2W (g)→ V (g)at 20 °C the following data were obtained: The rate law for this reaction is ________.</strong> A)Rate = -k[W]<sup>2</sup> B)Rate = -k[W] C)Rate = -k[W]<sup>0</sup> D)Rate = -k[W]<sup>-</sup><sup>1</sup>](https://storage.examlex.com/TB5644/11ea8554_7386_74d9_9aec_2d0261bd0484_TB5644_00.jpg) The rate law for this reaction is ________.
The rate law for this reaction is ________.
A)Rate = -k[W]2
B)Rate = -k[W]
C)Rate = -k[W]0
D)Rate = -k[W]-1
![<strong>In the following equation 2W (g)→ V (g)at 20 °C the following data were obtained: The rate law for this reaction is ________.</strong> A)Rate = -k[W]<sup>2</sup> B)Rate = -k[W] C)Rate = -k[W]<sup>0</sup> D)Rate = -k[W]<sup>-</sup><sup>1</sup>](https://storage.examlex.com/TB5644/11ea8554_7386_74d9_9aec_2d0261bd0484_TB5644_00.jpg) The rate law for this reaction is ________.
The rate law for this reaction is ________.A)Rate = -k[W]2
B)Rate = -k[W]
C)Rate = -k[W]0
D)Rate = -k[W]-1

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is true about the activation energy?
A)It may increase by decreasing the temperature.
B)It may decrease by decreasing the temperature.
C)It may increase by increasing the concentration of reactants.
D)It is practically independent of both temperature and concentration of reactants.
A)It may increase by decreasing the temperature.
B)It may decrease by decreasing the temperature.
C)It may increase by increasing the concentration of reactants.
D)It is practically independent of both temperature and concentration of reactants.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is true about a catalyst?
A)It increases the rate of a reaction.
B)It lowers the energy of activation.
C)It is not used up in the process.
D)All of the above are true.
A)It increases the rate of a reaction.
B)It lowers the energy of activation.
C)It is not used up in the process.
D)All of the above are true.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
The slow step in the multistep mechanism of a reaction ________.
A)controls the rate at which the products are formed
B)is the only step that involves the breaking of bonds
C)is equally significant in measuring the rate
D)is insignificant in the formation of products
A)controls the rate at which the products are formed
B)is the only step that involves the breaking of bonds
C)is equally significant in measuring the rate
D)is insignificant in the formation of products

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the reaction A + B → C. Doubling the concentration of A leads to doubling the rate, while doubling the concentration of B quadruples the rate. The rate equation is, therefore, ________.
A)Rate = -k[A][B]2
B)Rate = -k[A]
C)Rate = - k[A]2
D)Rate = -k[A]2[B]
A)Rate = -k[A][B]2
B)Rate = -k[A]
C)Rate = - k[A]2
D)Rate = -k[A]2[B]

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
A reaction follows the rate law: Rate = -k[A]2[B]1. The overall order is ________.
A)-1
B)0
C)2
D)3
A)-1
B)0
C)2
D)3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
The energy of activation may be affected by ________.
A)increasing the concentration of the reactants
B)higher temperature
C)decreasing the concentration of the reactants
D)the presence of a catalyst
A)increasing the concentration of the reactants
B)higher temperature
C)decreasing the concentration of the reactants
D)the presence of a catalyst

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
Generally in a biochemical reaction the first step involves ________.
A)the reaction of the enzyme with the active site of the substrate
B)the reaction of the substrate with the active site of the enzyme
C)the reaction of the substrate with the enzyme-substrate complex
D)the reaction of the enzyme with the enzyme-substrate complex
A)the reaction of the enzyme with the active site of the substrate
B)the reaction of the substrate with the active site of the enzyme
C)the reaction of the substrate with the enzyme-substrate complex
D)the reaction of the enzyme with the enzyme-substrate complex

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is not true when the temperature of the reaction mixture is decreased?
A)The average kinetic energy of both the reactant and product molecules is decreased.
B)The rate of the reaction is decreased.
C)The fraction of reactant molecules that have a low kinetic energies is increased.
D)The activation energy of the reaction is increased.
A)The average kinetic energy of both the reactant and product molecules is decreased.
B)The rate of the reaction is decreased.
C)The fraction of reactant molecules that have a low kinetic energies is increased.
D)The activation energy of the reaction is increased.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
The reaction A + B + C → Products follows the rate law: Rate = - k[A]1[B]-1[C]2. The overall rate of this reaction is ________.
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
A reaction follows the rate law: Rate = -k[A]3[B]-1. The overall order is ________.
A)-1
B)0
C)2
D)3
A)-1
B)0
C)2
D)3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is true about enzymes?
A)They speed up reactions in a selective fashion.
B)They are very large proteins.
C)They contain specifically shaped pockets that fit only specific reactant molecules.
D)All of the above are true.
A)They speed up reactions in a selective fashion.
B)They are very large proteins.
C)They contain specifically shaped pockets that fit only specific reactant molecules.
D)All of the above are true.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
A catalyst lowers the activation energy for a chemical reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
If the products have a higher energy than the reactants ________.
A)the reaction is exothermic
B)the reaction is endothermic
C)the reactants have less energy than the products
D)both B and C
A)the reaction is exothermic
B)the reaction is endothermic
C)the reactants have less energy than the products
D)both B and C

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
Given the reaction, 2A + B2 → 2AB, which of the following factors could increase the reaction rate?
I. decreasing the temperature
II.adding a catalyst
III.increasing the surface area of solid reactants
A)I and II
B)II and III
C)I and III
D)II And III
E)III only
I. decreasing the temperature
II.adding a catalyst
III.increasing the surface area of solid reactants
A)I and II
B)II and III
C)I and III
D)II And III
E)III only

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
An endothermic reaction usually leads to cooling.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
Adding a catalyst increases the rate of reaction by providing the additional energy needed to overcome a large energy of activation.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
For chemical reactions to occur at all, "old" bonds must be broken and "new" bonds must form.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
An exothermic reaction usually leads to a release in energy.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
For an exothermic reaction, the value for ΔErxn is a negative number.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
An exothermic reaction would feel cold.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
The rate constant of a reaction may be increased by ________.
A)increasing the concentration of reactants
B)increasing the temperature
C)adding a catalyst
D)all of the above
A)increasing the concentration of reactants
B)increasing the temperature
C)adding a catalyst
D)all of the above

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
The rate constant is dependant on the activation energy and reactant orientation.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Knowing the mechanism of a reaction is advantageous because one may influence its rate to make the reaction useful.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
The reaction energy profile can tell if a reaction is exothermic or endothermic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
It is possible to determine a given reaction's mechanism without performing any experiments of any kind.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
For an endothermic reaction, the value for ΔErxn is a negative number.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
The reaction: HCl + NaOH → H2O + NaCl is a substitution reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
In each of the following diagrams, the x-axis is the reaction coordinate and the y-axis is the potential energy. Which diagram corresponds to a reaction that has an activation energy of 35 kJ and an overall reaction energy of -110 kJ?
A)
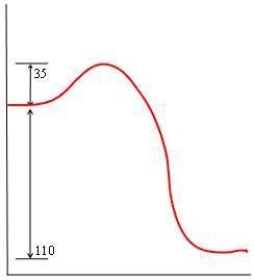
B)
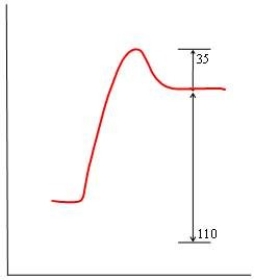
C)
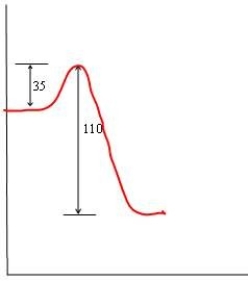
D)
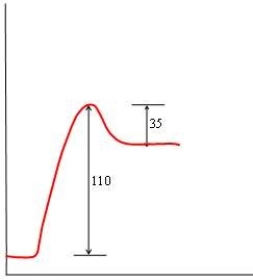
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
Reaction rates can be varied by changing either the concentrations of the reacting species or the temperature at which the reaction is carried out.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
When the heat of reaction is a positive number, the reaction is exothermic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
A lower energy of activation leads to more products favored in the reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
The magnitude (size)of the energy of activation for the reverse reaction is exactly identical as it is for the forward reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate a value for ΔErxn for a chemical reaction if the reactants have an energy of -400 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?
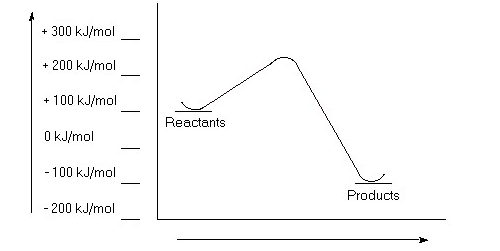
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?

a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
The greater the concentration of the reactants, the greater the rate of the reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
The value of Ea(reaction)is equal to the difference Ea(reactants)- Ea(products).

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
Based on the collision theory, adding a catalyst to a reaction increases the reaction rate.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
When the products are higher in energy than the reactants, the reaction is endothermic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
The reactants must collide with an energy ≥ the energy of activation for the reaction to occur.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
A high energy of activation leads to more products favored in the reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
Based on the collision theory, decreasing temperature for a reaction increases the reaction rate.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate a value for ΔErxn for a chemical reaction, if the reactants have an energy of +300 kJ/mol and the products have an energy of +100 kJ/mol. Is this reaction exothermic or endothermic?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
Match between columns

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
The rate determining step is always the fastest step.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
A catalyst can be recovered at the end of the reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
A catalyst need not be present in large quantities.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
Examine this reaction coordinate diagram and answer the questions that follow. 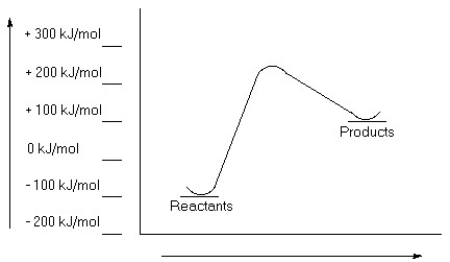 a)Estimate the value for the activation energy for this reaction.
a)Estimate the value for the activation energy for this reaction.
b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?
 a)Estimate the value for the activation energy for this reaction.
a)Estimate the value for the activation energy for this reaction.b)Calculate ΔErxn.
c)Is this reaction exothermic or endothermic?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
Industrial chemists often have their choice of catalysts to use when designing, for example, a factory that produces ammonia. Explain why biochemists studying life processes (such as protein metabolism)do not have the same variety of choices for which enzyme they would use.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
If the forward reaction is exothermic, the reverse is endothermic.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
Use the table below to answer the question that follows: ![Use the table below to answer the question that follows: The above data were obtained in a kinetic study of the reaction. a)Determine the orders x and y, assuming that rate = k[NO]<sup>x</sup>[Cl<sub>2</sub>]<sup>y</sup>. b)Determine the value for the rate constant, k, in Experiment 2. c)Determine the overall order of this reaction.](https://storage.examlex.com/TB5644/11ea8554_738a_456f_9aec_054782e874fc_TB5644_00.jpg)
The above data were obtained in a kinetic study of the reaction.
a)Determine the orders x and y, assuming that rate = k[NO]x[Cl2]y.
b)Determine the value for the rate constant, k, in Experiment 2.
c)Determine the overall order of this reaction.
![Use the table below to answer the question that follows: The above data were obtained in a kinetic study of the reaction. a)Determine the orders x and y, assuming that rate = k[NO]<sup>x</sup>[Cl<sub>2</sub>]<sup>y</sup>. b)Determine the value for the rate constant, k, in Experiment 2. c)Determine the overall order of this reaction.](https://storage.examlex.com/TB5644/11ea8554_738a_456f_9aec_054782e874fc_TB5644_00.jpg)
The above data were obtained in a kinetic study of the reaction.
a)Determine the orders x and y, assuming that rate = k[NO]x[Cl2]y.
b)Determine the value for the rate constant, k, in Experiment 2.
c)Determine the overall order of this reaction.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
In the reaction where Rate = [A]2, the rate is second order with respect to the reactant and second order overall.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
Match the rate of each reaction in the questions with the overall order that appears in the list below. 


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


