Deck 15: Electrolytes, Acids, and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 15: Electrolytes, Acids, and Bases
1
What is the pH of an aqueous solution having a hydrogen-ion concentration of 6.83 × 10-3 mol/L?
A)6.831
B)0.834
C)2.166
D)1.029
A)6.831
B)0.834
C)2.166
D)1.029
2.166
2
Which of the following produces only one H3O+ ion per molecule of acid when dissolved in water, although itself has other non-ionizable hydrogens?
A)hydrobromic
B)sulfurous
C)hypochlorous
D)acetic
A)hydrobromic
B)sulfurous
C)hypochlorous
D)acetic
acetic
3
Which of the following does not produce more than one H3O+ ion per molecule of acid when dissolved in water?
A)carbonic
B)sulfurous
C)phosphoric
D)acetic
A)carbonic
B)sulfurous
C)phosphoric
D)acetic
acetic
4
Which of the following could be considered an Arrhenius base?
A)HCl
B)CH3COOH
C)KOH
D)HF
A)HCl
B)CH3COOH
C)KOH
D)HF

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
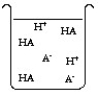
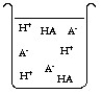
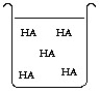
The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a weak acid in water?
A)
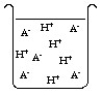
B)
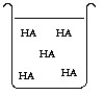
C)
D)Both A and C are weak acids in water.
A)

B)

C)

D)Both A and C are weak acids in water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following solutions would you expect not to conduct electricity?
A)NaCl in water
B)CO in water
C)HCl in water
D)H2SO4 in water
A)NaCl in water
B)CO in water
C)HCl in water
D)H2SO4 in water

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a triprotic acid when dissolved in water?
A)hydrobromic
B)sulfuric
C)hypochlorous
D)phosphoric
A)hydrobromic
B)sulfuric
C)hypochlorous
D)phosphoric

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a strong electrolyte when dissolved in distilled water?
A)sodium chloride
B)sucrose
C)acetone (nail polish remover)
D)ethyl alcohol
A)sodium chloride
B)sucrose
C)acetone (nail polish remover)
D)ethyl alcohol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a weak electrolyte when dissolved in distilled water?
A)phosphoric acid
B)nitric acid
C)potassium chloride
D)glucose
A)phosphoric acid
B)nitric acid
C)potassium chloride
D)glucose

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
What is the hydroxide-ion concentration of an aqueous solution having a pH of 4.72?
A)4.7 × 10-5 mol/L
B)1.9 × 10-14 mol/L
C)5.3 × 10-10 mol/L
D)1.9 × 10-5 mol/L
A)4.7 × 10-5 mol/L
B)1.9 × 10-14 mol/L
C)5.3 × 10-10 mol/L
D)1.9 × 10-5 mol/L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
What is the hydrogen-ion concentration of an aqueous solution having a pH of 4.72?
A)4.7 × 10-5 mol/L
B)4.7 mol/L
C)0.67 mol/L
D)1.9 × 10-5 mol/L
A)4.7 × 10-5 mol/L
B)4.7 mol/L
C)0.67 mol/L
D)1.9 × 10-5 mol/L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a non-electrolyte when dissolved in distilled water?
A)sodium chloride
B)hydrochloric acid
C)sodium hydroxide
D)ethyl alcohol
A)sodium chloride
B)hydrochloric acid
C)sodium hydroxide
D)ethyl alcohol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following could be considered an Arrhenius acid?
A)HCl
B)CH3COOH
C)NH4+
D)All of the above are Arrhenius acids.
A)HCl
B)CH3COOH
C)NH4+
D)All of the above are Arrhenius acids.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not a strong electrolyte when dissolved in distilled water?
A)potassium chloride
B)sulfuric acid
C)acetic acid
D)potassium hydroxide
A)potassium chloride
B)sulfuric acid
C)acetic acid
D)potassium hydroxide

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
What is the hydroxide-ion concentration of an aqueous solution having a hydrogen-ion concentration of 4.75 × 10-5 mol/L?
A)2.10 × 10-10 mol/L
B)5.25 × 10-10 mol/L
C)4.75 × 10-5 mol/L
D)-4.75 × 10-5 mol/L
A)2.10 × 10-10 mol/L
B)5.25 × 10-10 mol/L
C)4.75 × 10-5 mol/L
D)-4.75 × 10-5 mol/L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is not a weak electrolyte when dissolved in distilled water?
A)ammonia
B)acetic acid (vinegar)
C)potassium chloride
D)hydrogen fluoride
A)ammonia
B)acetic acid (vinegar)
C)potassium chloride
D)hydrogen fluoride

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
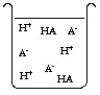
The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a strong acid in water?
A)
B)
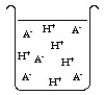
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following conjugate pairs would form a good buffer?
A)HCl/Cl-
B)OH-/H2O
C)NH3/NH4+
D)HNO3/NO3-
A)HCl/Cl-
B)OH-/H2O
C)NH3/NH4+
D)HNO3/NO3-

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a diprotic acid when dissolved in water?
A)hydrobromic
B)sulfuric
C)hypochlorous
D)phosphoric
A)hydrobromic
B)sulfuric
C)hypochlorous
D)phosphoric

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following produces more than one H3O+ ion per molecule of acid when dissolved in water?
A)hydrobromic
B)carbonic
C)hypochlorous
D)acetic
A)hydrobromic
B)carbonic
C)hypochlorous
D)acetic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
The pH of natural rainwater is close to ________.
A)5.6
B)6.3
C)7.0
D)8.2
A)5.6
B)6.3
C)7.0
D)8.2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following qualifies as a Bronsted-Lowry base?
A)CH4
B)NH3
C)SiH4
D)H2
A)CH4
B)NH3
C)SiH4
D)H2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
A change in pH from 10 to 7 ________.
A)increases the acidity 1000×
B)reduces the acidity 3000×
C)increases the acidity 3×
D)increases the acidity 3000×
A)increases the acidity 1000×
B)reduces the acidity 3000×
C)increases the acidity 3×
D)increases the acidity 3000×

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Which is a weak acid?
A)sulfuric
B)hydrochloric
C)nitric
D)carbonic
A)sulfuric
B)hydrochloric
C)nitric
D)carbonic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
The conjugate base of H2PO4- is ________.
A)H3PO4
B)HPO4-2
C)PO4-3
D)H4PO4+
A)H3PO4
B)HPO4-2
C)PO4-3
D)H4PO4+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Acidic solutions ________.
A)have a higher concentration of hydronium ions than hydroxide ions
B)have a lower concentration of hydronium ions than hydroxide ions
C)turn red litmus paper to blue
D)colorize phenolphthalein solutions
A)have a higher concentration of hydronium ions than hydroxide ions
B)have a lower concentration of hydronium ions than hydroxide ions
C)turn red litmus paper to blue
D)colorize phenolphthalein solutions

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following produces two OH- ions per molecule of base when dissolved in water?
A)potassium hydroxide
B)calcium hydroxide
C)aluminum hydroxide
D)ammonia
A)potassium hydroxide
B)calcium hydroxide
C)aluminum hydroxide
D)ammonia

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following qualifies as a Bronsted-Lowry acid?
A)KOH
B)NH3
C)Na+
D)NH4+
A)KOH
B)NH3
C)Na+
D)NH4+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following does not qualify as a Bronsted-Lowry base?
A)CH4
B)NH3
C)PH3
D)H2O
A)CH4
B)NH3
C)PH3
D)H2O

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
The pH of blood is approximately ________.
A)5.2
B)7.0
C)7.4
D)9.1
A)5.2
B)7.0
C)7.4
D)9.1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
Basic solutions ________.
A)have a higher concentration of hydronium ions than hydroxide ions
B)have a lower concentration of hydronium ions than hydroxide ions
C)turn blue litmus paper to pink
D)decolorize phenolphthalein solutions
A)have a higher concentration of hydronium ions than hydroxide ions
B)have a lower concentration of hydronium ions than hydroxide ions
C)turn blue litmus paper to pink
D)decolorize phenolphthalein solutions

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a strong acid?
A)acetic acid
B)hydrofluoric acid
C)phosphoric acid
D)hydrochloric acid
A)acetic acid
B)hydrofluoric acid
C)phosphoric acid
D)hydrochloric acid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
The conjugate base of ammonia is ________.
A)NH2-
B)NH-
C)N-3
D)NH4+
A)NH2-
B)NH-
C)N-3
D)NH4+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following can behave both as a Bronsted-Lowry acid and a Bronsted-Lowry base?
A)CH4
B)H2O
C)SiH4
D)H2
A)CH4
B)H2O
C)SiH4
D)H2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
The conjugate acid of H2PO4- is ________.
A)H3PO4
B)HPO4-2
C)PO4-3
D)H4PO4+
A)H3PO4
B)HPO4-2
C)PO4-3
D)H4PO4+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
When ammonia is dissolved in water, the ionized species are ________.
A)NH4+ and OH-
B)NH2- and H+
C)NH4+ and H-
D)NH2+ and H-
A)NH4+ and OH-
B)NH2- and H+
C)NH4+ and H-
D)NH2+ and H-

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Stomach juices have a pH close to ________.
A)1.5
B)2.8
C)4.5
D)7.0
A)1.5
B)2.8
C)4.5
D)7.0

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
The conjugate base of HCO3- is ________.
A)H2CO3
B)CO3-2
C)CO2-2
D)H3CO3+
A)H2CO3
B)CO3-2
C)CO2-2
D)H3CO3+

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
A drop of a full unit in the pH scale ________.
A)reduces the acidity 10×
B)increases the acidity 10×
C)reduces the acidity 2×
D)increases the acidity 2×
A)reduces the acidity 10×
B)increases the acidity 10×
C)reduces the acidity 2×
D)increases the acidity 2×

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following produces only one OH- ion per molecule of base when dissolved in water?
A)potassium hydroxide
B)calcium hydroxide
C)aluminum hydroxide
D)barium hydroxide
A)potassium hydroxide
B)calcium hydroxide
C)aluminum hydroxide
D)barium hydroxide

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Which is the most acidic solution?
A)a solution whose [H+] = 10-1
B)a solution whose [OH-] = 10-10
C)a solution whose pH = 0
D)All of the above have the same degree of acidity.
A)a solution whose [H+] = 10-1
B)a solution whose [OH-] = 10-10
C)a solution whose pH = 0
D)All of the above have the same degree of acidity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
A concentrated solution of a non-electrolyte such as glucose can be made to conduct electricity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Sodium chloride (NaCl)is considered an electrolyte because it dissolves in water into positive and negative ions.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is the weakest acid?
A)acetic acid, Ka = 1.8 × 10-5
B)nitrous acid, Ka = 4.5 × 10-4
C)hydrogen peroxide, Ka = 2.4 × 10-12
D)hypochlorous acid, Ka = 3.5 × 10-8
A)acetic acid, Ka = 1.8 × 10-5
B)nitrous acid, Ka = 4.5 × 10-4
C)hydrogen peroxide, Ka = 2.4 × 10-12
D)hypochlorous acid, Ka = 3.5 × 10-8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
In a solution of a strong acid in water, the hydrogen-ion concentration is approximately equal to the molarity of the acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
What are the ions formed in glucose, C6H12O6 ?
A)C6H11O6- + H+
B)C6H11O5+ + OH-
C)CO2 + H2O
D)No ions are formed.
A)C6H11O6- + H+
B)C6H11O5+ + OH-
C)CO2 + H2O
D)No ions are formed.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the weakest base?
A)ammonia, Kb = 1.7 × 10-5
B)aniline, Kb = 4.2 × 10-10
C)trimethylamine, Kb = 7.4 × 10-5
D)methylamine, Kb = 4.4 × 10-4
A)ammonia, Kb = 1.7 × 10-5
B)aniline, Kb = 4.2 × 10-10
C)trimethylamine, Kb = 7.4 × 10-5
D)methylamine, Kb = 4.4 × 10-4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Which substance is the most acidic?
A)coffee
B)milk
C)oven cleaner
D)baking soda
A)coffee
B)milk
C)oven cleaner
D)baking soda

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
The compound HCl is considered a Bronsted-Lowry base because it increases the concentration of H3O+ when it dissociates in water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Which is a strong base?
A)potassium hydroxide
B)ammonium hydroxide
C)aluminum hydroxide
D)fluoride ion
A)potassium hydroxide
B)ammonium hydroxide
C)aluminum hydroxide
D)fluoride ion

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
The base in the forward reaction NH3 + H2O → NH4+ + OH- is ________.
A)NH4+
B)H2O
C)NH3
D)OH-
A)NH4+
B)H2O
C)NH3
D)OH-

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
The hydrogen-ion concentration of a solution of pOH 10.5 is 3.16 × 10-4 mol/L (remember that pH + pOH = 14 for aqueous systems).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
The autodissociation of water gives pure water a pH of 4 and thus a higher concentration of H3O+ than OH-.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
An aqueous solution of pOH 5 is more basic than an aqueous solution of pOH 8.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
Which is the most basic solution?
A)a solution whose [H+] = 100
B)a solution whose [OH-] = 10-14
C)a solution whose pH = 0
D)All of the above have the same degree of basicity.
A)a solution whose [H+] = 100
B)a solution whose [OH-] = 10-14
C)a solution whose pH = 0
D)All of the above have the same degree of basicity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
"Buffered" solutions are resistant to pH changes, even when small amounts of strong acid or base are added to them.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is the strongest acid?
A)acetic acid, Ka = 1.8 × 10-5
B)nitrous acid, Ka = 4.5 × 10-4
C)hydrogen peroxide, Ka = 2.4 × 10-12
D)hypochlorous acid, Ka = 3.5 × 10-8
A)acetic acid, Ka = 1.8 × 10-5
B)nitrous acid, Ka = 4.5 × 10-4
C)hydrogen peroxide, Ka = 2.4 × 10-12
D)hypochlorous acid, Ka = 3.5 × 10-8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
The acid in the forward reaction HCO3- + H2O → H2CO3 + OH- is ________.
A)HCO3-
B)H2O
C)H2CO3
D)OH-
A)HCO3-
B)H2O
C)H2CO3
D)OH-

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
The carbonate (CO32-)/bicarbonate (HCO3-)pair represents a base and its conjugate acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is a weak base?
A)sodium hydroxide
B)ammonium hydroxide
C)lithium hydroxide
D)potassium hydroxide
A)sodium hydroxide
B)ammonium hydroxide
C)lithium hydroxide
D)potassium hydroxide

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
A saline solution will not conduct an electric current.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
H2PO4- is the conjugate acid of PO42-.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
PH3 cannot be described as a Bronsted-Lowry base.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
A solution of HCl/Cl- can act as a buffer.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
CH3CO2H has four acidic hydrogens.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Solid sodium chloride is a strong electrolyte.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
Water can serve both as a Bronsted-Lowry acid and Bronsted-Lowry base.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Milk's pH is slightly basic.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
Sulfuric acid is a diprotic acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Deionized water can conduct electricity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Approximately one out of every 555 million water molecules autoionize.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Hydrochloric, hypochlorous and hydrobromic acids produce only one H3O+ ion per molecule of acid when dissolved in water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Carbonic, phosphoric, and sulfuric acids produce two H3O+ ions per molecule of acid when dissolved in water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Sucrose (table sugar)is a strong electrolyte.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
An electrolyte is a substance that, when dissolved in water, yields a solution that conducts electric current.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
Aqueous salt solution is a strong electrolyte.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Arrhenius' definition of a base is an electrolyte that contains a metal and hydroxide ions that ionize when placed in water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
An aqueous solution of HCl is called hydrochloric acid.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
The pH of natural rainwater is 5.6 due to the carbon dioxide in the atmosphere.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Sodium hydroxide is a non-electrolyte.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


