Deck 2: Atoms and Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/158
Play
Full screen (f)
Deck 2: Atoms and Elements
1
Identify the smallest subatomic particle.
A) a neutron
B) an electron
C) a proton
D) an alpha particle
E) a nucleus
A) a neutron
B) an electron
C) a proton
D) an alpha particle
E) a nucleus
an electron
2
Which of the following statements is FALSE according to Dalton's Atomic Theory?
A) Atoms combine in simple whole number ratios to form compounds.
B) All atoms of chlorine have identical properties that distinguish them from other elements.
C) One carbon atom will combine with one oxygen atom to form a molecule of carbon monoxide.
D) Atoms of sodium do not change into another element during chemical reaction with chlorine.
E) An atom of nitrogen can be broken down into smaller particles that will still have the unique properties of nitrogen.
A) Atoms combine in simple whole number ratios to form compounds.
B) All atoms of chlorine have identical properties that distinguish them from other elements.
C) One carbon atom will combine with one oxygen atom to form a molecule of carbon monoxide.
D) Atoms of sodium do not change into another element during chemical reaction with chlorine.
E) An atom of nitrogen can be broken down into smaller particles that will still have the unique properties of nitrogen.
An atom of nitrogen can be broken down into smaller particles that will still have the unique properties of nitrogen.
3
Identify the description of an atom.
A) neutrons and electrons in nucleus; protons in orbitals
B) neutrons in nucleus; protons and electrons in orbitals
C) protons and neutrons in nucleus; electrons in orbitals
D) protons and electrons in nucleus; neutrons in orbitals
E) electrons in nucleus; protons and neutrons in orbitals
A) neutrons and electrons in nucleus; protons in orbitals
B) neutrons in nucleus; protons and electrons in orbitals
C) protons and neutrons in nucleus; electrons in orbitals
D) protons and electrons in nucleus; neutrons in orbitals
E) electrons in nucleus; protons and neutrons in orbitals
protons and neutrons in nucleus; electrons in orbitals
4
What does "X" represent in the following symbol? 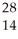 X
X
A) silicon
B) sulfur
C) zinc
D) ruthenium
E) nickel
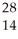 X
XA) silicon
B) sulfur
C) zinc
D) ruthenium
E) nickel

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the equipment that Millikan utilized to do his research.
A) cathode ray tube
B) light bulb
C) oscilloscope
D) oil atomizer, light source, microscope
E) electromagnetic field generator
A) cathode ray tube
B) light bulb
C) oscilloscope
D) oil atomizer, light source, microscope
E) electromagnetic field generator

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the equipment that Thomson utilized to do his research.
A) cathode ray tube
B) light bulb
C) oscilloscope
D) oil atomizer, light source, microscope
E) electromagnetic field generator
A) cathode ray tube
B) light bulb
C) oscilloscope
D) oil atomizer, light source, microscope
E) electromagnetic field generator

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the largest subatomic particle.
A) a neutron
B) an electron
C) a proton
D) an orbital
E) a nucleus
A) a neutron
B) an electron
C) a proton
D) an orbital
E) a nucleus

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
8
Determine the number of protons,neutrons,and electrons in the following: 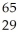 X
X
A) p+ = 36 n° = 29 e- = 36
B) p+ = 29 n° = 29 e- = 36
C) p+ = 36 n° = 36 e- = 29
D) p+ = 29 n° = 36 e- = 29
E) p+ = 29 n° = 36 e- = 36
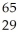 X
XA) p+ = 36 n° = 29 e- = 36
B) p+ = 29 n° = 29 e- = 36
C) p+ = 36 n° = 36 e- = 29
D) p+ = 29 n° = 36 e- = 29
E) p+ = 29 n° = 36 e- = 36

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
9
The atomic number is equal to
A) the number of the protons.
B) the sum of the number of the neutrons and electrons.
C) the sum of the number of protons, neutrons, and electrons.
D) the sum of the number of protons and neutrons.
A) the number of the protons.
B) the sum of the number of the neutrons and electrons.
C) the sum of the number of protons, neutrons, and electrons.
D) the sum of the number of protons and neutrons.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
10
In a chemical reaction,matter is neither created nor destroyed.Which law does this refer to?
A) Law of Definite Proportions
B) Law of the Conservation of Mass
C) Law of Modern Atomic Theory
D) Law of Multiple Proportions
E) First Law of Thermodynamics
A) Law of Definite Proportions
B) Law of the Conservation of Mass
C) Law of Modern Atomic Theory
D) Law of Multiple Proportions
E) First Law of Thermodynamics

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
11
All samples of a given compound,regardless of their source or how they were prepared,have the same proportions of their constituent elements.Which law does this refer to?
A) Law of Definite Proportions
B) Law of the Conservation of Mass
C) Law of Modern Atomic Theory
D) Law of Multiple Proportions
E) First Law of Thermodynamics
A) Law of Definite Proportions
B) Law of the Conservation of Mass
C) Law of Modern Atomic Theory
D) Law of Multiple Proportions
E) First Law of Thermodynamics

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an example of the law of multiple proportions?
A) A sample of chlorine is found to contain three times as much Cl-35 as Cl-37.
B) Two different compounds formed from carbon and oxygen have the following mass ratios: 1.33 g O: 1 g C and 2.66 g O: 1 g C.
C) Two different samples of table salt are found to have the same ratio of sodium to chlorine.
D) The atomic mass of bromine is found to be 79.90 amu.
E) Nitrogen dioxide always has a mass ratio of 2.28 g O: 1 g N.
A) A sample of chlorine is found to contain three times as much Cl-35 as Cl-37.
B) Two different compounds formed from carbon and oxygen have the following mass ratios: 1.33 g O: 1 g C and 2.66 g O: 1 g C.
C) Two different samples of table salt are found to have the same ratio of sodium to chlorine.
D) The atomic mass of bromine is found to be 79.90 amu.
E) Nitrogen dioxide always has a mass ratio of 2.28 g O: 1 g N.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
13
Determine the number of protons,neutrons,and electrons in the following: 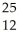 X
X
A) p+ = 12 n° = 25 e- = 12
B) p+ = 12 n° = 12 e- = 13
C) p+ = 12 n° = 13 e- = 12
D) p+ = 25 n° = 12 e- = 13
E) p+ = 12 n° = 13 e- = 25
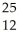 X
XA) p+ = 12 n° = 25 e- = 12
B) p+ = 12 n° = 12 e- = 13
C) p+ = 12 n° = 13 e- = 12
D) p+ = 25 n° = 12 e- = 13
E) p+ = 12 n° = 13 e- = 25

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
14
What element is defined by the following information? p+ = 20 n° = 20 e- = 20
A) zirconium
B) calcium
C) potassium
D) neon
E) argon
A) zirconium
B) calcium
C) potassium
D) neon
E) argon

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
15
What does "X" represent in the following symbol? 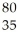 X
X
A) mercury
B) chlorine
C) scandium
D) bromine
E) selenium
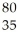 X
XA) mercury
B) chlorine
C) scandium
D) bromine
E) selenium

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
16
What element is defined by the following information? p+ = 11 n° = 12 e- = 11
A) sodium
B) vanadium
C) magnesium
D) titanium
E) selenium
A) sodium
B) vanadium
C) magnesium
D) titanium
E) selenium

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
17
Determine the number of protons,neutrons,and electrons in the following: 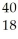 X
X
A) p+ = 18 n° = 18 e- = 22
B) p+ = 18 n° = 22 e- = 18
C) p+ = 22 n° = 18 e- = 18
D) p+ = 18 n° = 22 e- = 40
E) p+ = 40 n° = 22 e- = 18
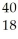 X
XA) p+ = 18 n° = 18 e- = 22
B) p+ = 18 n° = 22 e- = 18
C) p+ = 22 n° = 18 e- = 18
D) p+ = 18 n° = 22 e- = 40
E) p+ = 40 n° = 22 e- = 18

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
18
What element is defined by the following information? p+ = 17 n° = 20 e- = 17
A) calcium
B) rubidium
C) chlorine
D) neon
E) oxygen
A) calcium
B) rubidium
C) chlorine
D) neon
E) oxygen

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
19
When two elements form two different compounds,the masses of element B that combine with 1 g of element A can be expressed as a ratio of small whole numbers.Which law does this refer to?
A) Law of Definite Proportions
B) Law of the Conservation of Mass
C) Law of Modern Atomic Theory
D) Law of Multiple Proportions
E) First Law of Thermodynamics
A) Law of Definite Proportions
B) Law of the Conservation of Mass
C) Law of Modern Atomic Theory
D) Law of Multiple Proportions
E) First Law of Thermodynamics

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
20
The mass number is equal to
A) the sum of the number of the electrons and protons.
B) the sum of the number of the neutrons and electrons.
C) the sum of the number of protons, neutrons, and electrons.
D) the sum of the number of protons and neutrons.
A) the sum of the number of the electrons and protons.
B) the sum of the number of the neutrons and electrons.
C) the sum of the number of protons, neutrons, and electrons.
D) the sum of the number of protons and neutrons.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements is FALSE?
A) Anions are usually larger than their corresponding atom.
B) Metals tend to form cations.
C) Atoms are usually larger than their corresponding cation.
D) The halogens tend to form 1+ ions.
E) Nonmetals tend to gain electrons.
A) Anions are usually larger than their corresponding atom.
B) Metals tend to form cations.
C) Atoms are usually larger than their corresponding cation.
D) The halogens tend to form 1+ ions.
E) Nonmetals tend to gain electrons.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
22
Predict the charge that an aluminum ion would have.
A) 5-
B) 1+
C) 1-
D) 2+
E) 3+
A) 5-
B) 1+
C) 1-
D) 2+
E) 3+

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
23
The atomic mass for cadmium is
A) 48
B) 112.41
C) 40.08
D) 20
E) 64.411
A) 48
B) 112.41
C) 40.08
D) 20
E) 64.411

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the atomic mass of gallium if gallium has 2 naturally occurring isotopes with the following masses and natural abundances: Ga-69 68.9256 amu 60.11%
Ga-71 70.9247 amu 39.89%
A) 69.72 amu
B) 69.93 amu
C) 70.00 amu
D) 69.80 amu
E) 70.68 amu
Ga-71 70.9247 amu 39.89%
A) 69.72 amu
B) 69.93 amu
C) 70.00 amu
D) 69.80 amu
E) 70.68 amu

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
25
Give the symbol for silver.
A) S
B) Si
C) Ar
D) Ag
E) Sl
A) S
B) Si
C) Ar
D) Ag
E) Sl

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
26
What species is represented by the following information? p+ = 12 n° = 14 e- = 10
A) Si4+
B) Mg
C) Ne
D) Si
E) Mg2+
A) Si4+
B) Mg
C) Ne
D) Si
E) Mg2+

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is FALSE?
A) Halogens are very reactive elements.
B) The alkali metals are fairly unreactive.
C) Sulfur is a main group element.
D) Noble gases do not usually form ions.
E) Zn is a transition metal.
A) Halogens are very reactive elements.
B) The alkali metals are fairly unreactive.
C) Sulfur is a main group element.
D) Noble gases do not usually form ions.
E) Zn is a transition metal.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the atomic mass of silver if silver has 2 naturally occurring isotopes with the following masses and natural abundances: Ag-107 106.90509 amu 51.84%
Ag-109 108.90476 amu 48.46%
A) 107.90 amu
B) 108.00 amu
C) 107.79 amu
D) 108.32 amu
E) 108.19 amu
Ag-109 108.90476 amu 48.46%
A) 107.90 amu
B) 108.00 amu
C) 107.79 amu
D) 108.32 amu
E) 108.19 amu

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the green-yellowish gas that is used as a disinfecting agent.
A) chlorine
B) bromine
C) iodine
D) fluorine
A) chlorine
B) bromine
C) iodine
D) fluorine

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
30
Predict the charge that an ion formed from nitrogen would have.
A) 1-
B) 6+
C) 3-
D) 3+
E) 2-
A) 1-
B) 6+
C) 3-
D) 3+
E) 2-

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the instrument that is used to determine the mass of a molecule.
A) mass spectrometer
B) nuclear magnetic resonance spectrometer
C) infrared spectrometer
D) gas chromatograph
E) ultraviolet spectrophotometer
A) mass spectrometer
B) nuclear magnetic resonance spectrometer
C) infrared spectrometer
D) gas chromatograph
E) ultraviolet spectrophotometer

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
32
Ions differ in the number of
A) electrons.
B) neutrons.
C) protons.
D) neutrons and protons.
E) electrons and protons.
A) electrons.
B) neutrons.
C) protons.
D) neutrons and protons.
E) electrons and protons.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements about isotopes is TRUE?
A) Isotopes of the same element differ only in the number of electrons they contain.
B) An isotope of an atom with a larger number of neutrons is larger than an isotope of the same atom that contains fewer neutrons.
C) Isotopes of the same element have the same mass.
D) Isotopes of the same element don't usually have the same properties.
E) Some elements have 3 or more naturally occurring isotopes.
A) Isotopes of the same element differ only in the number of electrons they contain.
B) An isotope of an atom with a larger number of neutrons is larger than an isotope of the same atom that contains fewer neutrons.
C) Isotopes of the same element have the same mass.
D) Isotopes of the same element don't usually have the same properties.
E) Some elements have 3 or more naturally occurring isotopes.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the largest atom or ion of carbon.
A) p+ = 6 n° = 6 e- = 6
B) p+ = 6 n° = 7 e- = 6
C) p+ = 6 n° = 6 e- = 7
D) p+ = 6 n° = 6 e- = 5
A) p+ = 6 n° = 6 e- = 6
B) p+ = 6 n° = 7 e- = 6
C) p+ = 6 n° = 6 e- = 7
D) p+ = 6 n° = 6 e- = 5

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
35
Semiconductors are
A) metalloids.
B) noble gases.
C) nonmetals.
D) metals.
A) metalloids.
B) noble gases.
C) nonmetals.
D) metals.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following does NOT describe a nonmetal?
A) tends to gain electrons
B) found in the upper right hand corner of the periodic table
C) poor conductor of electricity
D) nonmetals are generally unreactive
E) poor conductor of heat
A) tends to gain electrons
B) found in the upper right hand corner of the periodic table
C) poor conductor of electricity
D) nonmetals are generally unreactive
E) poor conductor of heat

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
37
What species is represented by the following information? p+ = 47 n° = 62 e- = 46
A) Ag+
B) Nd
C) Pd
D) Ag
E) Pd+
A) Ag+
B) Nd
C) Pd
D) Ag
E) Pd+

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
38
What species is represented by the following information? p+ = 17 n° = 18 e- = 18
A) Cl
B) Cl-
C) Ar
D) Ar+
E) Kr
A) Cl
B) Cl-
C) Ar
D) Ar+
E) Kr

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements about subatomic particles is TRUE?
A) A neutral atom contains the same number of protons and electrons.
B) Protons have about the same mass as electrons.
C) Electrons make up most of the mass of an atom.
D) Protons and neutrons have opposite, but equal in magnitude, charges.
E) Neutrons and electrons are found in the nucleus of an atom.
A) A neutral atom contains the same number of protons and electrons.
B) Protons have about the same mass as electrons.
C) Electrons make up most of the mass of an atom.
D) Protons and neutrons have opposite, but equal in magnitude, charges.
E) Neutrons and electrons are found in the nucleus of an atom.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following does NOT describe a metal?
A) good conductor of heat
B) good conductor of electricity
C) tends to gain electrons
D) forms ionic compounds with nonmetals
E) found on the left side of the periodic table
A) good conductor of heat
B) good conductor of electricity
C) tends to gain electrons
D) forms ionic compounds with nonmetals
E) found on the left side of the periodic table

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
41
Gallium has an atomic mass of 69.723 amu.The Ga-69 (68.926 amu)is 60.11%.What is the amu of the other isotope?
A) 70.924 amu
B) 70.928 amu
C) 70.932 amu
D) 70.920 amu
A) 70.924 amu
B) 70.928 amu
C) 70.932 amu
D) 70.920 amu

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
42
Identify how the atomic mass of an element is determined.
A) average of ions
B) average of isotopic masses
C) average of radioactive particles
D) average of crude weights
E) average of protons
A) average of ions
B) average of isotopic masses
C) average of radioactive particles
D) average of crude weights
E) average of protons

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the atomic mass of element "X",if it has 2 naturally occurring isotopes with the following masses and natural abundances: X-45 44.8776 amu 32.88%
X-47 46.9443 amu 67.12%
A) 46.26 amu
B) 45.91 amu
C) 46.34 amu
D) 46.84 amu
E) 44.99 amu
X-47 46.9443 amu 67.12%
A) 46.26 amu
B) 45.91 amu
C) 46.34 amu
D) 46.84 amu
E) 44.99 amu

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
44
How many iron atoms are contained in 354 g of iron?
A) 2.62 × 1025 Fe atoms
B) 2.13 × 1026 Fe atoms
C) 4.69 × 1024 Fe atoms
D) 3.82 × 1024 Fe atoms
E) 9.50 × 1022 Fe atoms
A) 2.62 × 1025 Fe atoms
B) 2.13 × 1026 Fe atoms
C) 4.69 × 1024 Fe atoms
D) 3.82 × 1024 Fe atoms
E) 9.50 × 1022 Fe atoms

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the mass (in kg)of 4.87 × 1025 atoms of Zn.
A) 5.29 kg
B) 1.89 kg
C) 8.09 kg
D) 1.24 kg
E) 1.09 kg
A) 5.29 kg
B) 1.89 kg
C) 8.09 kg
D) 1.24 kg
E) 1.09 kg

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
46
How many moles of Cs are contained in 595 kg of Cs?
A) 2.23 × 102 moles Cs
B) 4.48 × 103 moles Cs
C) 7.91 × 104 moles Cs
D) 1.26 × 103 moles Cs
E) 5.39 × 102 moles Cs
A) 2.23 × 102 moles Cs
B) 4.48 × 103 moles Cs
C) 7.91 × 104 moles Cs
D) 1.26 × 103 moles Cs
E) 5.39 × 102 moles Cs

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
47
How many phosphorus atoms are contained in 158 kg of phosphorus?
A) 3.07 × 1027 phosphorus atoms
B) 2.95 × 1027 phosphorus atoms
C) 3.25 × 1028 phosphorus atoms
D) 1.18 × 1024 phosphorus atoms
E) 8.47 × 1024 phosphorus atoms
A) 3.07 × 1027 phosphorus atoms
B) 2.95 × 1027 phosphorus atoms
C) 3.25 × 1028 phosphorus atoms
D) 1.18 × 1024 phosphorus atoms
E) 8.47 × 1024 phosphorus atoms

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the radioactive particle.
A) a beta particle
B) an anion
C) a molecule
D) an electron
E) a mole
A) a beta particle
B) an anion
C) a molecule
D) an electron
E) a mole

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
49
Identify the discovery that Millikan made.
A) the mass of a proton
B) the charge of a proton
C) the charge of a single electron
D) the mass of a beta particle
E) molecules
A) the mass of a proton
B) the charge of a proton
C) the charge of a single electron
D) the mass of a beta particle
E) molecules

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
50
How many xenon atoms are contained in 2.36 moles of xenon?
A) 3.92 × 1024 xenon atoms
B) 2.55 × 1023 xenon atoms
C) 1.42 × 1024 xenon atoms
D) 7.91 × 1025 xenon atoms
E) 1.87 × 1026 xenon atoms
A) 3.92 × 1024 xenon atoms
B) 2.55 × 1023 xenon atoms
C) 1.42 × 1024 xenon atoms
D) 7.91 × 1025 xenon atoms
E) 1.87 × 1026 xenon atoms

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the element that has an atomic number of 40.
A) neon
B) calcium
C) zirconium
D) bromine
E) gold
A) neon
B) calcium
C) zirconium
D) bromine
E) gold

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
52
Calculate the mass (in ng)of 2.33 × 1020 atoms of oxygen.
A) 6.19 × 106 ng
B) 1.62 × 107 ng
C) 2.25 × 103 ng
D) 3.73 × 106 ng
E) 4.69 × 107 ng
A) 6.19 × 106 ng
B) 1.62 × 107 ng
C) 2.25 × 103 ng
D) 3.73 × 106 ng
E) 4.69 × 107 ng

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
53
An atom of 13C contains ________ protons.
A) 6
B) 19
C) 7
D) 9
E) 13
A) 6
B) 19
C) 7
D) 9
E) 13

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
54
Give the symbol for potassium.
A) K
B) P
C) Po
D) Ka
E) Pt
A) K
B) P
C) Po
D) Ka
E) Pt

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
55
Rutherford proposed the
A) atomic bomb.
B) hydroelectric.
C) First Law of Conservation.
D) theory of explosives.
E) nuclear theory.
A) atomic bomb.
B) hydroelectric.
C) First Law of Conservation.
D) theory of explosives.
E) nuclear theory.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
56
How many moles of Kr are contained in 398 mg of Kr?
A) 4.75 × 10-3 moles Kr
B) 33.4 moles Kr
C) 2.11 × 10-4 moles Kr
D) 2.99 × 10-3 moles Kr
E) 1.19 × 10-4 moles Kr
A) 4.75 × 10-3 moles Kr
B) 33.4 moles Kr
C) 2.11 × 10-4 moles Kr
D) 2.99 × 10-3 moles Kr
E) 1.19 × 10-4 moles Kr

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
57
What mass (in mg)does 2.63 moles of nickel have?
A) 44.8 mg
B) 2.23 × 104 mg
C) 129 mg
D) 3.56 × 105 mg
E) 1.54 × 105 mg
A) 44.8 mg
B) 2.23 × 104 mg
C) 129 mg
D) 3.56 × 105 mg
E) 1.54 × 105 mg

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the discovery that Thomson made.
A) a neutron
B) an electron
C) the charge of a single electron
D) an alpha particle
E) anions
A) a neutron
B) an electron
C) the charge of a single electron
D) an alpha particle
E) anions

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
59
Silver has an atomic mass of 107.868 amu.The Ag-109 isotope (108.905 amu)is 48.16%.What is the amu of the other isotope?
A) 106.905 amu
B) 106.908 amu
C) 106.903 amu
D) 106.911 amu
A) 106.905 amu
B) 106.908 amu
C) 106.903 amu
D) 106.911 amu

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the charges of the protons,neutrons,and electrons.
A) protons +1, neutrons 0, electrons -1
B) protons 0, neutrons -1, electrons 0
C) protons -1, neutrons -1, electrons 0
D) protons 0, neutrons 0, electrons 0
E) protons +1, neutrons +1, electrons +1
A) protons +1, neutrons 0, electrons -1
B) protons 0, neutrons -1, electrons 0
C) protons -1, neutrons -1, electrons 0
D) protons 0, neutrons 0, electrons 0
E) protons +1, neutrons +1, electrons +1

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
61
What does "X" represent in the following symbol? 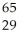 X
X
A) dysprosium
B) nickel
C) terbium
D) silicon
E) copper
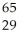 X
XA) dysprosium
B) nickel
C) terbium
D) silicon
E) copper

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
62
An atom that has an atomic number of 38 and a mass number of 88 is an isotope of an atom that has
A) an atomic number of 39 and a mass number of 88.
B) an atomic number of 38 and a mass number of 86.
C) 50 neutrons and 38 protons.
D) 50 protons and 38 neutrons.
A) an atomic number of 39 and a mass number of 88.
B) an atomic number of 38 and a mass number of 86.
C) 50 neutrons and 38 protons.
D) 50 protons and 38 neutrons.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
63
Identify an anion.
A) an atom that has lost an electron
B) an atom that has gained an electron
C) an atom that has lost a proton
D) an atom that has gained a neutron and a proton
A) an atom that has lost an electron
B) an atom that has gained an electron
C) an atom that has lost a proton
D) an atom that has gained a neutron and a proton

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
64
How many protons are in nickel?
A) 28
B) 30
C) 31
D) 30.7
E) 58.7
A) 28
B) 30
C) 31
D) 30.7
E) 58.7

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
65
Which element has the chemical symbol,Sn?
A) samarium
B) lead
C) tin
D) tungsten
E) selenium
A) samarium
B) lead
C) tin
D) tungsten
E) selenium

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
66
An atom of 25Mg contains ________ electrons.
A) 25
B) 37
C) 13
D) 23
E) 12
A) 25
B) 37
C) 13
D) 23
E) 12

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
67
Identify a cation.
A) an atom that has lost an electron
B) an atom that has gained an electron
C) an atom that has lost a proton
D) an atom that has gained a neutron
A) an atom that has lost an electron
B) an atom that has gained an electron
C) an atom that has lost a proton
D) an atom that has gained a neutron

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
68
What is the chemical symbol for thallium?
A) Ti
B) Tl
C) Tm
D) Th
E) Tr
A) Ti
B) Tl
C) Tm
D) Th
E) Tr

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
69
How many electrons does the Se2- ion possess?
A) 32
B) 36
C) 4
D) 0
E) 34
A) 32
B) 36
C) 4
D) 0
E) 34

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
70
How many protons does the As3- ion possess?
A) 30
B) 36
C) 4
D) 8
E) 33
A) 30
B) 36
C) 4
D) 8
E) 33

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
71
How many electrons are in each neutral atom of magnesium?
A) 12
B) 13
C) 14
D) 12.3
E) 24.3
A) 12
B) 13
C) 14
D) 12.3
E) 24.3

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
72
How many protons (p)and neutrons (n)are in an atom of 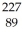 Ac?
Ac?
A) 89 p, 138 n
B) 89 p, 227 n
C) 138 p, 89 n
D) 227 p, 89 n
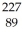 Ac?
Ac?A) 89 p, 138 n
B) 89 p, 227 n
C) 138 p, 89 n
D) 227 p, 89 n

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
73
Isotopes differ in the number of
A) gamma particles.
B) electrons.
C) compounds.
D) neutrons.
E) neutrons and protons.
A) gamma particles.
B) electrons.
C) compounds.
D) neutrons.
E) neutrons and protons.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following represent isotopes? A: 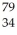 X B:
X B: 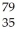 X C:
X C: 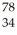 X D:
X D: 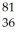 X
X
A) A and D
B) A and C
C) B and D
D) C and D
E) all of the above
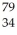 X B:
X B: 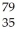 X C:
X C: 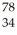 X D:
X D: 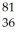 X
XA) A and D
B) A and C
C) B and D
D) C and D
E) all of the above

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
75
Which element has the chemical symbol,Rn?
A) radium
B) radon
C) rhenium
D) rhodium
A) radium
B) radon
C) rhenium
D) rhodium

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
76
An ion has 26 protons,29 neutrons,and 23 electrons.The symbol for the ion is ________.
A) (55Fe3+)
B) (55Fe3- )
C) (52Cu3+)
D) (52Cu3-)
E) (55V3-)
A) (55Fe3+)
B) (55Fe3- )
C) (52Cu3+)
D) (52Cu3-)
E) (55V3-)

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
77
How many neutrons are in manganese-54?
A) 25
B) 30
C) 29
D) 29.4
E) 54.9
A) 25
B) 30
C) 29
D) 29.4
E) 54.9

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
78
The atomic number of an atom of 130Xe is
A) 184.
B) 54.
C) 76.
D) 122.
E) 130.
A) 184.
B) 54.
C) 76.
D) 122.
E) 130.

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
79
What is the chemical symbol for molybdenum?
A) Ma
B) Au
C) Mo
D) K
E) Mb
A) Ma
B) Au
C) Mo
D) K
E) Mb

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck
80
What is the chemical symbol for carbon?
A) Co
B) Cr
C) C
D) Ca
E) Cb
A) Co
B) Cr
C) C
D) Ca
E) Cb

Unlock Deck
Unlock for access to all 158 flashcards in this deck.
Unlock Deck
k this deck


