Deck 7: The Quantum-Mechanical Model of the Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/135
Play
Full screen (f)
Deck 7: The Quantum-Mechanical Model of the Atom
1
Food can be cooked by ________ radiation.
A) ultraviolet
B) gamma
C) microwave
D) x-ray
E) radio
A) ultraviolet
B) gamma
C) microwave
D) x-ray
E) radio
microwave
2
A type of energy embodied in oscillating electric and magnetic fields is called
A) infrared radiation.
B) microwave radiation.
C) magnetism.
D) electricity.
E) electromagnetic radiation.
A) infrared radiation.
B) microwave radiation.
C) magnetism.
D) electricity.
E) electromagnetic radiation.
electromagnetic radiation.
3
When waves of equal amplitude from two sources are out of phase when they interact,it is called
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.
destructive interference.
4
Which of the following colors of electromagnetic radiation has the shortest wavelength?
A) blue
B) violet
C) orange
D) green
E) yellow
A) blue
B) violet
C) orange
D) green
E) yellow

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
5
________ is/are used to image bones and internal organs.
A) Ultraviolet light
B) Gamma rays
C) Microwaves
D) X-rays
E) Radio waves
A) Ultraviolet light
B) Gamma rays
C) Microwaves
D) X-rays
E) Radio waves

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
6
A sunburn is caused by overexposure to ________ radiation.
A) ultraviolet
B) gamma
C) microwave
D) x-ray
E) radio
A) ultraviolet
B) gamma
C) microwave
D) x-ray
E) radio

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
7
________,used to destroy molecules within unwanted cells such as cancer cells,are called ionizing radiation.
A) Beta and gamma rays
B) Alpha and beta rays
C) Microwave and infrared
D) X-rays and gamma rays
E) Visible and microwave
A) Beta and gamma rays
B) Alpha and beta rays
C) Microwave and infrared
D) X-rays and gamma rays
E) Visible and microwave

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
8
The distance between adjacent crests is called
A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.
A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
9
When a wave encounters an obstacle or a slit that is comparable in size to its wavelength,it bends around it.This characteristic is called
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following visible colors of light has the highest frequency?
A) green
B) red
C) blue
D) yellow
E) orange
A) green
B) red
C) blue
D) yellow
E) orange

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the wavelength (in nm)of the blue light emitted by a mercury lamp with a frequency of 6.88 × 1014 Hz.
A) 229 nm
B) 436 nm
C) 206 nm
D) 485 nm
E) 675 nm
A) 229 nm
B) 436 nm
C) 206 nm
D) 485 nm
E) 675 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate the energy of the orange light emitted,per photon,by a neon sign with a frequency of 4.89 × 1014 Hz.
A) 3.09 × 10-19 J
B) 6.14 × 10-19 J
C) 3.24 × 10-19 J
D) 1.63 × 10-19 J
E) 5.11 × 10-19 J
A) 3.09 × 10-19 J
B) 6.14 × 10-19 J
C) 3.24 × 10-19 J
D) 1.63 × 10-19 J
E) 5.11 × 10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
13
The vertical height of a wave is called
A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.
A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following visible colors of light has the longest wavelength?
A) blue
B) green
C) yellow
D) red
E) violet
A) blue
B) green
C) yellow
D) red
E) violet

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the frequency of the green light emitted by a hydrogen atom with a wavelength of 486.1 nm.
A) 1.46 × 1014 s-1
B) 6.86 × 1014 s-1
C) 4.33 × 1014 s-1
D) 6.17 × 1014 s-1
E) 1.62 × 1014 s-1
A) 1.46 × 1014 s-1
B) 6.86 × 1014 s-1
C) 4.33 × 1014 s-1
D) 6.17 × 1014 s-1
E) 1.62 × 1014 s-1

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
16
The heat that is felt from a hot object is called ________ radiation.
A) ultraviolet
B) gamma
C) infrared
D) x-ray
E) microwave
A) ultraviolet
B) gamma
C) infrared
D) x-ray
E) microwave

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
17
The number of cycles that pass through a stationary point is called
A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.
A) wavelength.
B) amplitude.
C) frequency.
D) area.
E) median.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
18
When waves of equal amplitude from two sources are in phase when they interact,it is called
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.
A) destructive interference.
B) diffraction.
C) constructive interference.
D) effusion.
E) amplitude.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following types of electromagnetic radiation has the lowest frequency?
A) yellow
B) blue
C) orange
D) green
E) purple
A) yellow
B) blue
C) orange
D) green
E) purple

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the energy of the green light emitted,per photon,by a mercury lamp with a frequency of 5.49 × 1014 Hz.
A) 2.75 × 10-19 J
B) 3.64 × 10-19 J
C) 5.46 × 10-19 J
D) 1.83 × 10-19 J
E) 4.68 × 10-19 J
A) 2.75 × 10-19 J
B) 3.64 × 10-19 J
C) 5.46 × 10-19 J
D) 1.83 × 10-19 J
E) 4.68 × 10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
21
Determine the longest wavelength of light required to remove an electron from a sample of potassium metal,if the binding energy for an electron in K is 1.76 × 103 kJ/mol.
A) 147 nm
B) 68.0 nm
C) 113 nm
D) 885 nm
E) 387 nm
A) 147 nm
B) 68.0 nm
C) 113 nm
D) 885 nm
E) 387 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
22
When an electric current is passed through a tube containing hydrogen,hydrogen atoms absorb some of the electrical energy and reemit it as a ________ color.
A) white
B) yellow
C) violet
D) blue
E) red
A) white
B) yellow
C) violet
D) blue
E) red

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
23
When an electric current is passed through a tube containing helium,helium atoms absorb some of the electrical energy and reemit it as a ________ color.
A) white
B) yellow
C) violet
D) blue
E) red
A) white
B) yellow
C) violet
D) blue
E) red

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
24
Identify the color of a flame test for barium.
A) violet
B) red
C) white
D) yellow
E) blue
A) violet
B) red
C) white
D) yellow
E) blue

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the color of a flame test for lithium.
A) violet
B) red
C) white
D) yellow
E) blue
A) violet
B) red
C) white
D) yellow
E) blue

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the energy of the red light emitted by a neon atom with a wavelength of 703.2 nm.
A) 3.54 × 10-19 J
B) 4.27 × 10-19 J
C) 2.34 × 10-19 J
D) 6.45 × 10-19 J
E) 2.83 × 10-19 J
A) 3.54 × 10-19 J
B) 4.27 × 10-19 J
C) 2.34 × 10-19 J
D) 6.45 × 10-19 J
E) 2.83 × 10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
27
How many photons are contained in a flash of green light (525 nm)that contains 189 kJ of energy?
A) 5.67 × 1023 photons
B) 2.01 × 1024 photons
C) 1.25 × 1031 photons
D) 4.99 × 1023 photons
E) 7.99 × 1030 photons
A) 5.67 × 1023 photons
B) 2.01 × 1024 photons
C) 1.25 × 1031 photons
D) 4.99 × 1023 photons
E) 7.99 × 1030 photons

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
28
What total energy (in kJ)is contained in 1.0 mol of photons,all with a frequency of 2.75 × 1014 Hz?
A) 182 kJ
B) 219 kJ
C) 457 kJ
D) 326 kJ
E) 110 kJ
A) 182 kJ
B) 219 kJ
C) 457 kJ
D) 326 kJ
E) 110 kJ

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements is TRUE?
A) The emission spectrum of a particular element is always the same and can be used to identify the element.
B) Part of the Bohr model proposed that electrons in the hydrogen atom are located in "stationary states" or particular orbits around the nucleus.
C) The uncertainty principle states that we can never know both the exact location and speed of an electron.
D) An orbital is the volume in which we are most likely to find an electron.
E) All of the above are true.
A) The emission spectrum of a particular element is always the same and can be used to identify the element.
B) Part of the Bohr model proposed that electrons in the hydrogen atom are located in "stationary states" or particular orbits around the nucleus.
C) The uncertainty principle states that we can never know both the exact location and speed of an electron.
D) An orbital is the volume in which we are most likely to find an electron.
E) All of the above are true.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
30
Determine the mass of a ball with a wavelength of 3.45 × 10-34 m and a velocity of 6.55 m/s.
A) 0.293 g
B) 12.6 g
C) 293 g
D) 346 g
E) 3.41 g
A) 0.293 g
B) 12.6 g
C) 293 g
D) 346 g
E) 3.41 g

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the wavelength of an electron (m = 9.11 × 10-28 g)moving at 3.66 × 106 m/s.
A) 1.99 × 10-10 m
B) 5.03 × 10-10 m
C) 1.81 × 10-10 m
D) 5.52 × 10-9 m
E) 2.76 × 10-9 m
A) 1.99 × 10-10 m
B) 5.03 × 10-10 m
C) 1.81 × 10-10 m
D) 5.52 × 10-9 m
E) 2.76 × 10-9 m

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
32
Determine the velocity of a medicine ball (m = 10.0 kg)with a wavelength of 1.33 × 10-35 m.
A) 8.81 m/s
B) 12.3 m/s
C) 2.21 m/s
D) 4.98 m/s
E) 6.44 m/s
A) 8.81 m/s
B) 12.3 m/s
C) 2.21 m/s
D) 4.98 m/s
E) 6.44 m/s

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
33
It is possible to determine the ionization energy for hydrogen using the Bohr equation.Calculate the ionization energy for an atom of hydrogen,making the assumption that ionization is the transition from n = 1 to n = ∞.
A) -2.18 × 10-18 J
B) +2.18 × 10-18 J
C) +4.59 × 10-18 J
D) -4.59 × 10-18 J
E) +4.36 × 10-18 J
A) -2.18 × 10-18 J
B) +2.18 × 10-18 J
C) +4.59 × 10-18 J
D) -4.59 × 10-18 J
E) +4.36 × 10-18 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the color of a flame test for sodium.
A) violet
B) red
C) white
D) yellow
E) blue
A) violet
B) red
C) white
D) yellow
E) blue

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
35
When an electric current is passed through a tube containing mercury,mercury atoms absorb some of the electrical energy and reemit it as a ________ color.
A) white
B) yellow
C) violet
D) blue
E) red
A) white
B) yellow
C) violet
D) blue
E) red

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the wavelength of a baseball (m = 155 g)moving at 32.5 m/s.
A) 7.60 × 10-36 m
B) 1.32 × 10-34 m
C) 2.15 × 10-32 m
D) 2.68 × 10-34 m
E) 3.57 × 10-32 m
A) 7.60 × 10-36 m
B) 1.32 × 10-34 m
C) 2.15 × 10-32 m
D) 2.68 × 10-34 m
E) 3.57 × 10-32 m

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
37
When an electric current is passed through a tube containing neon,neon atoms absorb some of the electrical energy and reemit it as a ________ color.
A) white
B) yellow
C) violet
D) blue
E) red
A) white
B) yellow
C) violet
D) blue
E) red

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
38
Determine the shortest frequency of light required to remove an electron from a sample of Ti metal if the binding energy of titanium is 3.14 × 103 kJ/mol.
A) 7.87 × 1015 Hz
B) 4.74 × 1015 Hz
C) 2.11 × 1015 Hz
D) 1.27 × 1015 Hz
E) 6.19 × 1015 Hz
A) 7.87 × 1015 Hz
B) 4.74 × 1015 Hz
C) 2.11 × 1015 Hz
D) 1.27 × 1015 Hz
E) 6.19 × 1015 Hz

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the color of a flame test for potassium.
A) violet
B) red
C) white
D) yellow
E) blue
A) violet
B) red
C) white
D) yellow
E) blue

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the energy of the violet light emitted by a hydrogen atom with a wavelength of 410.1 nm.
A) 4.85 × 10-19 J
B) 2.06 × 10-19 J
C) 1.23 × 10-19 J
D) 8.13 × 10-19 J
E) 5.27 x 10-19 J
A) 4.85 × 10-19 J
B) 2.06 × 10-19 J
C) 1.23 × 10-19 J
D) 8.13 × 10-19 J
E) 5.27 x 10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
41
Determine the end (final)value of n in a hydrogen atom transition,if the electron starts in n = 1 and the atom absorbs a photon of light with an energy of 2.044 × 10-18 J.
A) 3
B) 4
C) 2
D) 5
E) 6
A) 3
B) 4
C) 2
D) 5
E) 6

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
42
If two electrons in the same atom have the same value of l,they are
A) in the same sublevel, but not necessarily in the same level.
B) in the same level, but different sublevel.
C) in the same orbital.
D) in different levels and in different shaped orbitals.
E) none of the above.
A) in the same sublevel, but not necessarily in the same level.
B) in the same level, but different sublevel.
C) in the same orbital.
D) in different levels and in different shaped orbitals.
E) none of the above.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following statements is TRUE?
A) The principal quantum number (n) describes the shape of an orbital.
B) The angular momentum quantum number (l) describes the the size and energy associated with an orbital.
C) The magnetic quantum number (ml) describes the orientation of the orbital.
D) An orbital is the path that an electron follows during its movement in an atom.
E) All of the above are true.
A) The principal quantum number (n) describes the shape of an orbital.
B) The angular momentum quantum number (l) describes the the size and energy associated with an orbital.
C) The magnetic quantum number (ml) describes the orientation of the orbital.
D) An orbital is the path that an electron follows during its movement in an atom.
E) All of the above are true.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the energy change associated with the transition from 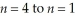 in the hydrogen atom.
in the hydrogen atom.
A) +4.89 × 10-18 J
B) +1.64 × 10-18 J
C) -6.12 × 10-18 J
D) +3.55 × 10-18 J
E) -2.04 × 10-18 J
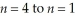 in the hydrogen atom.
in the hydrogen atom.A) +4.89 × 10-18 J
B) +1.64 × 10-18 J
C) -6.12 × 10-18 J
D) +3.55 × 10-18 J
E) -2.04 × 10-18 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the energy change associated with the transition from 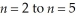 in the hydrogen atom.
in the hydrogen atom.
A) -2.18 × 10-19 J
B) +6.54 × 10-19 J
C) +4.58 × 10-19 J
D) -1.53 × 10-19 J
E) +3.76 × 10-19 J
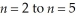 in the hydrogen atom.
in the hydrogen atom.A) -2.18 × 10-19 J
B) +6.54 × 10-19 J
C) +4.58 × 10-19 J
D) -1.53 × 10-19 J
E) +3.76 × 10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements is TRUE?
A) We can sometimes know the exact location and speed of an electron at the same time.
B) All orbitals in a given atom are roughly the same size.
C) Since electrons have mass, we must always consider them to have particle properties and never wavelike properties.
D) Atoms are roughly spherical because when all of the different shaped orbitals are overlapped, they take on a spherical shape.
E) All of the above are true.
A) We can sometimes know the exact location and speed of an electron at the same time.
B) All orbitals in a given atom are roughly the same size.
C) Since electrons have mass, we must always consider them to have particle properties and never wavelike properties.
D) Atoms are roughly spherical because when all of the different shaped orbitals are overlapped, they take on a spherical shape.
E) All of the above are true.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following quantum numbers describes the shape of an orbital?
A) principal quantum number
B) magnetic quantum number
C) spin quantum number
D) Schrödinger quantum number
E) angular momentum quantum number
A) principal quantum number
B) magnetic quantum number
C) spin quantum number
D) Schrödinger quantum number
E) angular momentum quantum number

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
48
What are the possible orbitals for n = 3?
A) s, p, d
B) s, p, d, f
C) s
D) s, p
A) s, p, d
B) s, p, d, f
C) s
D) s, p

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the wavelength of light associated with the transition from n = 1 to n = 3 in the hydrogen atom.
A) 103 nm
B) 155 nm
C) 646 nm
D) 971 nm
E) 136 nm
A) 103 nm
B) 155 nm
C) 646 nm
D) 971 nm
E) 136 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the frequency of light associated with the transition from n = 2 to n = 3 in the hydrogen atom.
A) 2.19 × 1014 s-1
B) 5.59 × 1014 s-1
C) 4.57 × 1014 s-1
D) 1.79 × 1014 s-1
E) 3.28 × 1014 s-1
A) 2.19 × 1014 s-1
B) 5.59 × 1014 s-1
C) 4.57 × 1014 s-1
D) 1.79 × 1014 s-1
E) 3.28 × 1014 s-1

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the end (final)value of n in a hydrogen atom transition,if the electron starts in 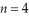 and the atom emits a photon of light with a wavelength of 486 nm.
and the atom emits a photon of light with a wavelength of 486 nm.
A) 1
B) 5
C) 3
D) 4
E) 2
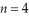 and the atom emits a photon of light with a wavelength of 486 nm.
and the atom emits a photon of light with a wavelength of 486 nm.A) 1
B) 5
C) 3
D) 4
E) 2

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
52
What value of l is represented by a f orbital?
A) 1
B) 2
C) 0
D) 3
A) 1
B) 2
C) 0
D) 3

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following quantum numbers describes the size and energy of an orbital?
A) magnetic quantum number
B) principal quantum number
C) angular momentum quantum number
D) spin quantum number
E) Schrödinger quantum number
A) magnetic quantum number
B) principal quantum number
C) angular momentum quantum number
D) spin quantum number
E) Schrödinger quantum number

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
54
For n = 3,what are the possible values for l?
A) 0
B) 0, 1
C) 0, 1, 2
D) 0, 1, 2, 3
A) 0
B) 0, 1
C) 0, 1, 2
D) 0, 1, 2, 3

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
55
It is possible to determine the ionization energy for hydrogen using the Bohr equation.Calculate the ionization energy (in kJ)for a mole of hydrogen atoms,making the assumption that ionization is the transition from n = 1 to n = ∞.
A) 7.62 × 103 kJ
B) 2.76 × 103 kJ
C) 1.31 × 103 kJ
D) 3.62 × 103 kJ
E) 5.33 × 103 kJ
A) 7.62 × 103 kJ
B) 2.76 × 103 kJ
C) 1.31 × 103 kJ
D) 3.62 × 103 kJ
E) 5.33 × 103 kJ

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
56
What value of l is represented by a d orbital?
A) 1
B) 2
C) 0
D) 3
A) 1
B) 2
C) 0
D) 3

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following quantum numbers describes the orientation of an orbital?
A) magnetic quantum number
B) principal quantum number
C) angular momentum quantum number
D) spin quantum number
E) Schrödinger quantum number
A) magnetic quantum number
B) principal quantum number
C) angular momentum quantum number
D) spin quantum number
E) Schrödinger quantum number

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
58
Determine the energy change associated with the transition from 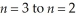 in the hydrogen atom.
in the hydrogen atom.
A) +3.03 × 10-19 J
B) -1.82 × 10-19 J
C) +5.51 × 10-19 J
D) -3.03 × 10-19 J
E) +2.69 × 10-19 J
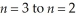 in the hydrogen atom.
in the hydrogen atom.A) +3.03 × 10-19 J
B) -1.82 × 10-19 J
C) +5.51 × 10-19 J
D) -3.03 × 10-19 J
E) +2.69 × 10-19 J

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
59
Determine the end (final)value of n in a hydrogen atom transition,if the electron starts in n = 2 and the atom absorbs a photon of light with a frequency of 4.57 × 1014 Hz.
A) 3
B) 1
C) 4
D) 6
E) 7
A) 3
B) 1
C) 4
D) 6
E) 7

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
60
How many different values of l are possible in the third principal level?
A) 1
B) 2
C) 3
D) 0
E) 4
A) 1
B) 2
C) 3
D) 0
E) 4

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate the wavelength (in nm)of the red light emitted by a neon sign with a frequency of 4.76 × 1014 Hz.
A) 630 nm
B) 159 nm
C) 476 nm
D) 776 nm
E) 176 nm
A) 630 nm
B) 159 nm
C) 476 nm
D) 776 nm
E) 176 nm

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
62
An FM radio station broadcasts electromagnetic radiation at a frequency of 101.2 MHz.The wavelength of this radiation is ________ m.
A) 2.964 × 106
B) 2.964
C) 3.036 × 1016
D) 3.036 × 1010
E) 0.3373
A) 2.964 × 106
B) 2.964
C) 3.036 × 1016
D) 3.036 × 1010
E) 0.3373

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the orbital. 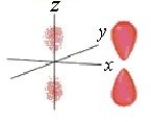
A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital

A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the orbital. 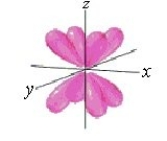
A) d orbital
B) dxy orbital
C) px orbital
D) f orbital
E) py orbital

A) d orbital
B) dxy orbital
C) px orbital
D) f orbital
E) py orbital

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following occurs as the wavelength of a photon increases?
A) The frequency decreases.
B) The energy increases.
C) The speed decreases.
D) Planck's constant increases.
E) None of the above occurs as the wavelength of a photon increases.
A) The frequency decreases.
B) The energy increases.
C) The speed decreases.
D) Planck's constant increases.
E) None of the above occurs as the wavelength of a photon increases.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate the frequency of the red light emitted by a neon sign with a wavelength of 680 nm.
A) 2.27 × 1014 s-1
B) 3.80 × 1014 s-1
C) 4.41 × 1014 s-1
D) 5.05 × 1014 s-1
E) 3.40 × 1014 s-1
A) 2.27 × 1014 s-1
B) 3.80 × 1014 s-1
C) 4.41 × 1014 s-1
D) 5.05 × 1014 s-1
E) 3.40 × 1014 s-1

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
67
Identify the orbital. 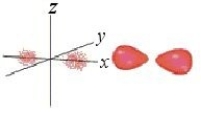
A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital

A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
68
Place the following types of electromagnetic radiation in order of increasing wavelength. visible light x-rays microwaves
A) x-rays < microwaves < visible light
B) microwaves < visible light < x-rays
C) microwaves < x-rays < visible light
D) visible light < x-rays < microwaves
E) x-rays < visible light < microwaves
A) x-rays < microwaves < visible light
B) microwaves < visible light < x-rays
C) microwaves < x-rays < visible light
D) visible light < x-rays < microwaves
E) x-rays < visible light < microwaves

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
69
Identify the orbital. 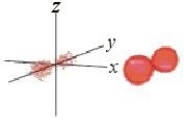
A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital

A) pz orbital
B) dxy orbital
C) px orbital
D) dyz orbital
E) py orbital

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
70
Identify the orbital. 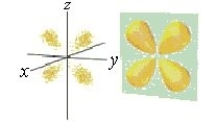
A) d orbital
B) dxy orbital
C) px orbital
D) f orbital
E) py orbital

A) d orbital
B) dxy orbital
C) px orbital
D) f orbital
E) py orbital

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
71
Identify the color that has a wavelength of 730 nm.
A) blue
B) green
C) red
D) yellow
E) magenta
A) blue
B) green
C) red
D) yellow
E) magenta

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
72
Electromagnetic radiation with a wavelength of 640 nm appears as orange light to the human eye.The frequency of this light is ________ s-1.
A) 4.688 × 1014
B) 4.688 × 105
C) 1.920 × 102
D) 1.920 × 1011
E) 2.133 × 10-15
A) 4.688 × 1014
B) 4.688 × 105
C) 1.920 × 102
D) 1.920 × 1011
E) 2.133 × 10-15

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
73
Place the following types of electromagnetic radiation in order of increasing frequency. x-rays radio waves gamma rays
A) radio waves < x-rays < gamma rays
B) gamma rays < x-rays < radio waves
C) radio waves < gamma rays < x-rays
D) gamma rays < radio waves < x-rays
E) x-rays < gamma rays < radio waves
A) radio waves < x-rays < gamma rays
B) gamma rays < x-rays < radio waves
C) radio waves < gamma rays < x-rays
D) gamma rays < radio waves < x-rays
E) x-rays < gamma rays < radio waves

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
74
Electromagnetic radiation with a wavelength of 745 nm appears as red light to the human eye.The energy of one photon of this light is ________ J.
A) 1.48 × 10-31
B) 2.67 × 10-28
C) 2.67 × 10-19
D) 1.48 × 10-22
E) 3.75 × 1018
A) 1.48 × 10-31
B) 2.67 × 10-28
C) 2.67 × 10-19
D) 1.48 × 10-22
E) 3.75 × 1018

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
75
Identify the color that has a wavelength of 545 nm.
A) blue
B) green
C) red
D) yellow
E) violet
A) blue
B) green
C) red
D) yellow
E) violet

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
76
Identify the color that has a wavelength of 480 nm.
A) blue
B) green
C) red
D) yellow
E) violet
A) blue
B) green
C) red
D) yellow
E) violet

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
77
Place the following types of electromagnetic radiation in order of decreasing energy. visible light radio waves infrared light
A) radio waves > infrared light > visible light
B) visible light > infrared light > radio waves
C) radio waves > visible light > infrared light
D) visible light > radio waves > infrared light
E) infrared light > radio waves > visible light
A) radio waves > infrared light > visible light
B) visible light > infrared light > radio waves
C) radio waves > visible light > infrared light
D) visible light > radio waves > infrared light
E) infrared light > radio waves > visible light

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following occurs as the energy of a photon increases?
A) The frequency decreases.
B) The speed increases.
C) Planck's constant increases.
D) The frequency increases.
E) None of the above occurs as the energy of a photon increases.
A) The frequency decreases.
B) The speed increases.
C) Planck's constant increases.
D) The frequency increases.
E) None of the above occurs as the energy of a photon increases.

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
79
On the electromagnetic spectrum,visible light is immediately between two other wavelengths.Name them.
A) infrared and gamma ray
B) radio and gamma ray
C) gamma ray and ultraviolet x-ray
D) microwave and ultraviolet x-ray
E) infrared and ultraviolet
A) infrared and gamma ray
B) radio and gamma ray
C) gamma ray and ultraviolet x-ray
D) microwave and ultraviolet x-ray
E) infrared and ultraviolet

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck
80
During a thunderstorm,you see a flash of lightning.Five seconds later,you hear the thunder.How far away is the storm?
A) 1 yard
B) 10 miles
C) 1 mile
D) 6 miles
E) 12 miles
A) 1 yard
B) 10 miles
C) 1 mile
D) 6 miles
E) 12 miles

Unlock Deck
Unlock for access to all 135 flashcards in this deck.
Unlock Deck
k this deck


